Home > Information > Memorandum of Cooperation on Decentralized Clinical Trials between the Department of Medical Services and the National Cancer Center Japan.
Memorandum of Cooperation on Decentralized Clinical Trials between the Department of Medical Services and the National Cancer Center Japan.
June 27, 2023
Department of Medical Services, Thailand
National Cancer Center, Japan
In Japanese
Abstract
- Until now, cross-border Decentralized Clinical Trials (DCTs) have never been conducted worldwide mainly due to the need for a local national medical license. By concluding the memorandum of cooperation (MoU) between the Department of Medical Services (DMS) Thailand and National Cancer Center (NCC) Japan, the two parties agreed to promote innovative international clinical trials, such as DCTs.
- For the first time ever, a cross-national DCT has been planned, involving patients from Thailand participating online. This was made possible by granting temporary medical license in Thailand to the medical oncologists at NCC conducting the DCT in Japan.
- It opens the way for patients living in Thailand to be able to participate conveniently in clinical trials conducted in Japan, and it is also possible for Japan to work with Thailand to achieve early patient enrollment.
Overview
DMS (Director General, Thongchai Keeratihutthayakorn; Director of National Cancer Institute, Napa Siriwiwattnakul) and NCC (President, Hitoshi Nakagama; Hospital Director, Kazuaki Shimada) concluded a MoU on the promotion of innovative international clinical trials including DCT.
The MoU defines that both DMS and NCC work together to promote international collaboration, that NCC proposes and conducts multiple international clinical trials, and that DMS has established a committee to promote this MoU and resolves the issues. In particular, the MoU included the agreement to issue a Thai temporary medical license to NCC’s medical oncologists for the conduct of DCT between the two countries, which set out to work together to achieve the world's first cross-border DCT.
Background
Since 2020, NCC has been promoting the Asian Clinical Trials Network for Cancers Project (ATLAS project) funded by the Japan Agency for Medical Research and Development (AMED) to build establish a robust clinical trial network in Asia, including Thailand. In 2021, NCC has established its first overseas office was established in Bangkok, Thailand, and cooperation was strengthened by concluding a memorandum of cooperation with many Thai medical institutions and academic organizations.
Under the ATLAS project, several international clinical trials have been conducted with Thailand and other Asian countries. However, the cost of international clinical trials is higher than those domestically conducted in Japan, leading to many unsuccessful cases where study proposals were rejected due to funding issues. DCTs have the potential to significantly reduce clinical trial costs compared to conventional trials, since clinical trial management such as monitoring is conducted remotely. Based on these backgrounds, DMS and NCC had an intensive discussion to extend the DCT scheme internationally to Thailand.
One major obstacle for realize cross-border DCT is the issue of local medical licensing. Traditionally, medical care laws in various countries assume that patients and doctors are located within the same country. However, the recent advancement of digital technology has challenged this premise. While online cross-border second opinions have been sometimes accepted as an activity without the medical care, DCTs require conducting clinical trial-related medical care across borders. Therefore, Japanese physicians need to obtain a Thai medical license to conduct DCTs for patients living in Thailand.
To address this issue, collaborative efforts with the DMS and other stakeholders have resulted in issuing temporary medical licenses to medical oncologists at NCC conducting DCT. These medical licenses are provided and the medical care for DCTs is made under the supervision of Thai physicians. This solution has overcome the legal obstacles and paved the way for the world’s first cross-border DCTs.
Cross-border Decentralized Clinical Trials
In the planned cross-border DCTs, NCC and designated Thai medical institutions (partner hospitals) will conclude a trial-specific contract, where NCC delegates the necessary tests for the trials to the partner sites. The test results are then shared with NCC through the internet platform. The medical care related to the trial is carried out online by medical oncologists at NCC, under the supervision of the principal investigator at the partner hospital. The prescription and control of the investigational drug will be entrusted to partner institutions in Thailand. The basic structure of the planned cross-border DCT is illustrated in the figure below.
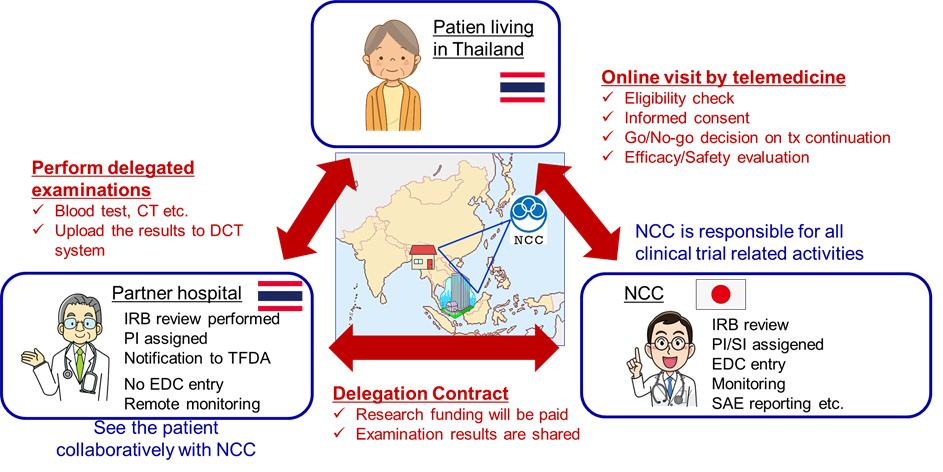
The three advantages of implementing this scheme are as follows:
- Patients living in Thailand, who have had limited opportunities to participate in clinical trials thus far, can conveniently take part in trials conducted in Japan.
- Even in trials where it is challenging to recruit enough patients solely in Japan, early patient accrual becomes possible by enrolling patients from Thailand.
- By sharing clinical trial records and materials online, monitoring for Thai medical institutions can be simplified, leading to significant reduced costs and resources required for the trials.
By introducing this new DCT scheme, patient enrollment across borders becomes easier, faster, and more cost-effective.
Contents of cooperation memorandum (Abstract)
- DMS and NCC will work jointly to pursue potential funding opportunities for international collaborative research.
- NCC offers opportunities for capacity development to Thai medical institutions by proposing and coordinating multiple international clinical trials and promoting DCTs.
- DMS will organize a committee with the relevant stakeholders to promote this memorandum of cooperation, create a multi-institutional trial network in Thailand, and conduct multi-international collaborative research.
- An investigator-initiated DCT will be conducted as a demonstration project, and patients will be taken care jointly by the Thai principal investigators and Japanese medical oncologists. The two parties will optimize procedures for the conduct of international DCTs through these demonstration projects.
- To give temporary medical license for the conduct of DCTs to medical oncologists at NCC and to allow them to perform medical care for patients living in Thailand under the supervision of a Thai principal investigator.
- Regular meetings will be held between the two countries to promote the Memorandum of Cooperation.
Future perspectives
NCC and designated Thai partner institutions will conduct a cross-border DCT as a demonstration project in collaboration with pharmaceutical companies.
The aim of this project is to streamline procedures on DCTs between the two countries and explore methods that will allow for more international clinical trials to be conducted in a simple, fast, and cost-effective manner.
As the cross-border DCT is definitely a pioneering initiatives, the two countries will work on together and eventually expand this scheme to other regions and develop an efficient system for implementing clinical trials throughout Asia.
ATLAS Project
ATLAS Project is a project to build a robust Asian cancer trials network, which has been implemented since September 2020 under the support of the Japan Agency for Medical Research and Development (AMED). In order to improve the presence of Asia as the third pillar of pharmaceutical and medical device development alongside the U.S. and Europe, NCC aims to create a continuous Asian cancer clinical trial network that will facilitate various international clinical trials of Asia, by Asia and for Asia. Currently, eight countries including Japan, Korea, Taiwan, the Philippines, Vietnam, Thailand, Malaysia, and Singapore are collaboratively developing the network participating in multiple Asian international clinical trials, with NCC providing the necessary infrastructure for high-quality clinical trials and training for international clinical trials.
Research funding
Asian Cancer Trials Network Project funded by Japan Agency for Medical Research and Development (AMED) 23lk0201009j0001, FY2023-5
Principal investigator, Kazuaki Shimada, MD PhD
Introduction of DCT to Rare Cancer Platform Trial. Asian Cancer Trials Network Project funded by Japan Agency for Medical Research and Development (AMED) 23lk0221182h0001, FY2023-6
Principal investigator, Kenichi Nakamura, MD PhD MBA
Contacts
Inquiry about research
Kenichi Nakamura
Department of International Clinical Development, National Cancer Center Hospital
Email: kennakam●ncc.go.jp
Public relations service
Public Relations & Strategy Office, Strategic Planning Bureau, National Cancer Center
E-mail: ncc-admin●ncc.go.jp

