Home > Information > press release > Molecular Mechanisms of Chromosomal Instability and Anti-Tumor Immune Activation Induced by DNA Replication Stress
Molecular Mechanisms of Chromosomal Instability and Anti-Tumor Immune Activation Induced by DNA Replication Stress-Expectations for the Development of Novel Cancer Immunotherapy Targeting DNA Replication Stress-
November 29, 2023
National Cancer Center
In Japanese
Highlights
- Molecular mechanisms of CDC7 inhibitor-mediated chromosomal instability and anti-tumor immune activation though DNA replication stress*1.
- We have determined that a CDC7 inhibitor TAK-931*2 enhances antitumor activity of immune checkpoint inhibitors*3 in preclinical mouse models.
- Cancer immunotherapies that target DNA replication stress therapeutically could be a potential novel therapeutic strategy.
Summary
An international collaborative research team led by Dr. Akihiro Ohashi, Head of Division of Collaborative Research and Development at Exploratory Oncology Research and Clinical Center (Director: Toshihiko Doi, Kashiwa-shi, Chiba), National Cancer Center (President: Hitoshi Nakagama Chuo-ku, Tokyo) has elucidated the molecular mechanisms underlying DNA replication stress-induced chromosome instability*4 and anti-tumor immune activation. The research team found that TAK-931, a novel cell division cycle 7 (CDC7) kinase inhibitor, a molecular target drug candidate developed by Takeda , strongly induces chromosome instability in cancer cells via DNA replication stress, and CDC7 inhibitor-induced chromosomal instability activates anti-tumor immunity to sensitize immune checkpoint inhibitors in preclinical models. These findings suggest novel cancer immunotherapy targeting DNA replication stress might be a good mechanistic strategy for enhancing the anti-tumor activity of immune checkpoint blockade. This research was published in "Nature Communications" (November 18,2023).
Background
While immune checkpoint inhibitors (ICIs) are currently used as standard therapy in a wide range of cancer types, recent studies reveal that there are many ICI-resistant cancers with "low immunogenicity". Aiming to develop novel therapies or combination therapies which would be effective for these ICI-resistant cancers, we have experimentally tested a novel therapeutic hypothesis with a novel CDC7 inhibitor, TAK-931, focusing on the "functional interaction between DNA replication stress (RS) and ICI sensitivity.
Study method/results
1. CDC7 inhibitor-mediated RS causes chromosomal instability in cancer cells, activating inflammatory responses.
We revealed that the CDC7 inhibitor TAK-931 strongly induces RS-mediated chromosomal instability in cancer cells, exhibiting cellular senescent morphology, such as flattened and enlarged characteristics, to activate intracellular inflammatory responses (Figure 1A). Gene expression analysis also confirmed that the expression of inflammatory cytokines and chemokines*5 such as IL6, CCL5, and CXCL10 were significantly upregulated in the flattened and enlarged cells in treatment with TAK-931 compared to negative control (Figure 1B). Since inflammatory cytokines and chemokines are known to activate immune cells and promote tumor infiltration, we conducted a study in numbers of preclinical models to prove the hypothesis that "induction of chromosomal instability in cancer cells by CDC7 inhibitors may enhance anti-tumor immune effects" (Figure 1C).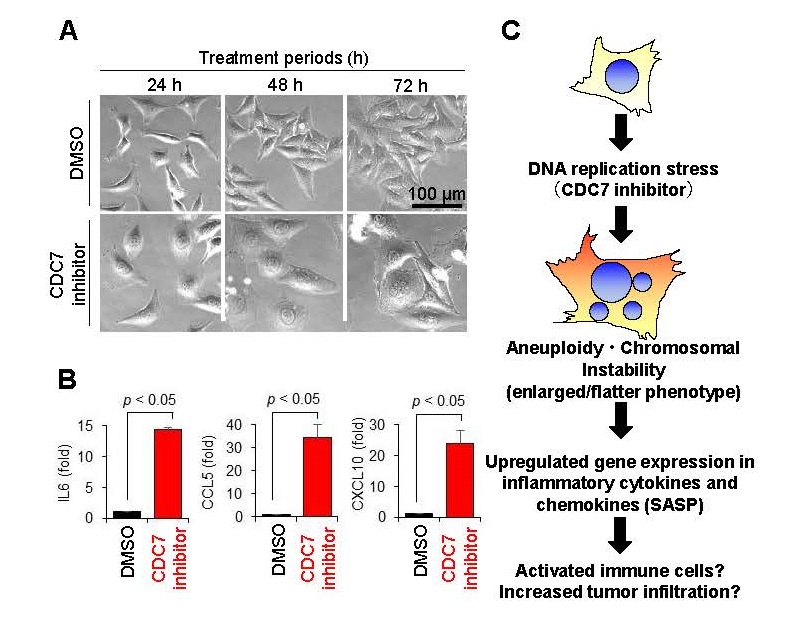
Fig.1 Cellular morphology and inflammatory response in CDC7 inhibitor-treated cells
A. Morphology of HeLa cells after treatment with CDC7 inhibitor TAK-931
B. Gene expression of IL6, CCL5, and CXCL10 in CDC7 inhibitor TAK-931 treated cells
C. Hypothesized pharmacological effects of CDC7 inhibitor on RS and intracellular inflammatory response
2. DNA replication stress (RS) induced by CDC7 inhibitors causes intratumor infiltration of immune cells.
To investigate whether CDC7 inhibitor-mediated RS promotes immune cell infiltration into tumors, we conducted preclinical studies in a mouse subcutaneously allografted tumor model. Mice allografted with mouse tumors were orally administered the CDC7 inhibitor TAK-931 and a negative control for 5 to 6 days, and tumor-infiltrating immune cells in allografts were visualized by immunohistochemical staining with the indicated immune cell markers (Figure 2A). As shown in Figure 2B, T cells (Cd3-positive cells), cytotoxic T cells (Cd8-positive cells), helper T cells (Cd4-positive cells), and dendritic cells (Cd11c-positive cells) were infiltrated more abundantly in the allografts treated with CDC7 inhibitor (Figure 2B, lower panel) compared to negative controls (Figure 2B, upper panel).
We also determined effects of CDC7 inhibitor mediated RS on immunogenicity in preclinical models. Rechallenge experiments (Fig. 3A) revealed that CDC7 inhibitor-treated mice, that had fully cleared an initial tumor challenge, rejected the allografted tumors, demonstrating enhanced antitumor immunity against the rechallenging tumor (Fig. 3B). Thus, CDC7 inhibitor-mediated RS and chromosomal instability in cancer cells appear to activate anti-tumor immune responses in the preclinical models.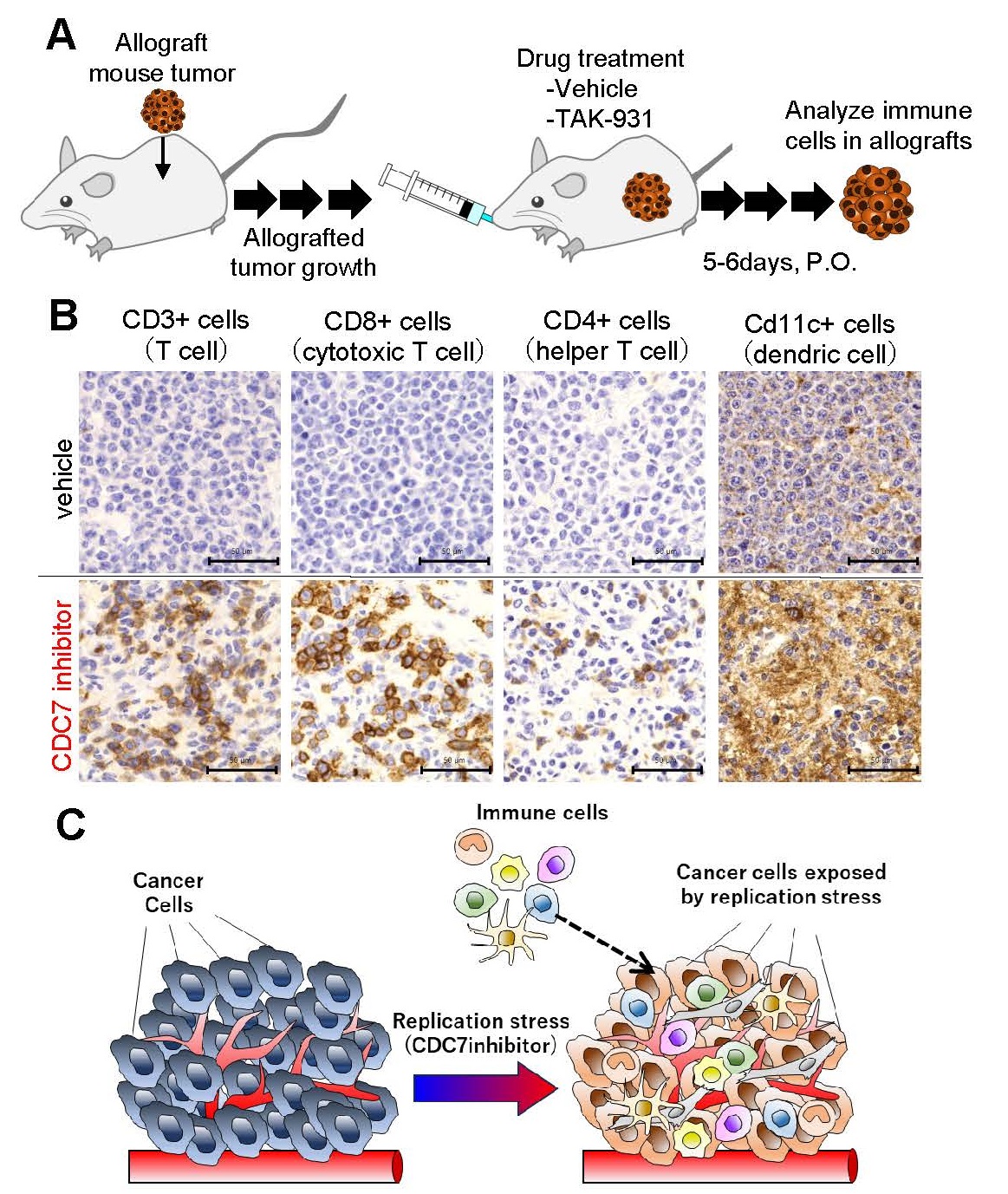
Fig. 2 Experiments of immune cells infiltrating into tumors using mouse models
A. Experimental design of intratumor infiltration of immune cellsB. Immunohistochemical staining using mouse transplanted tumor
C. Summary of RS and intratumor infiltration of immune cells by CDC7 inhibitor
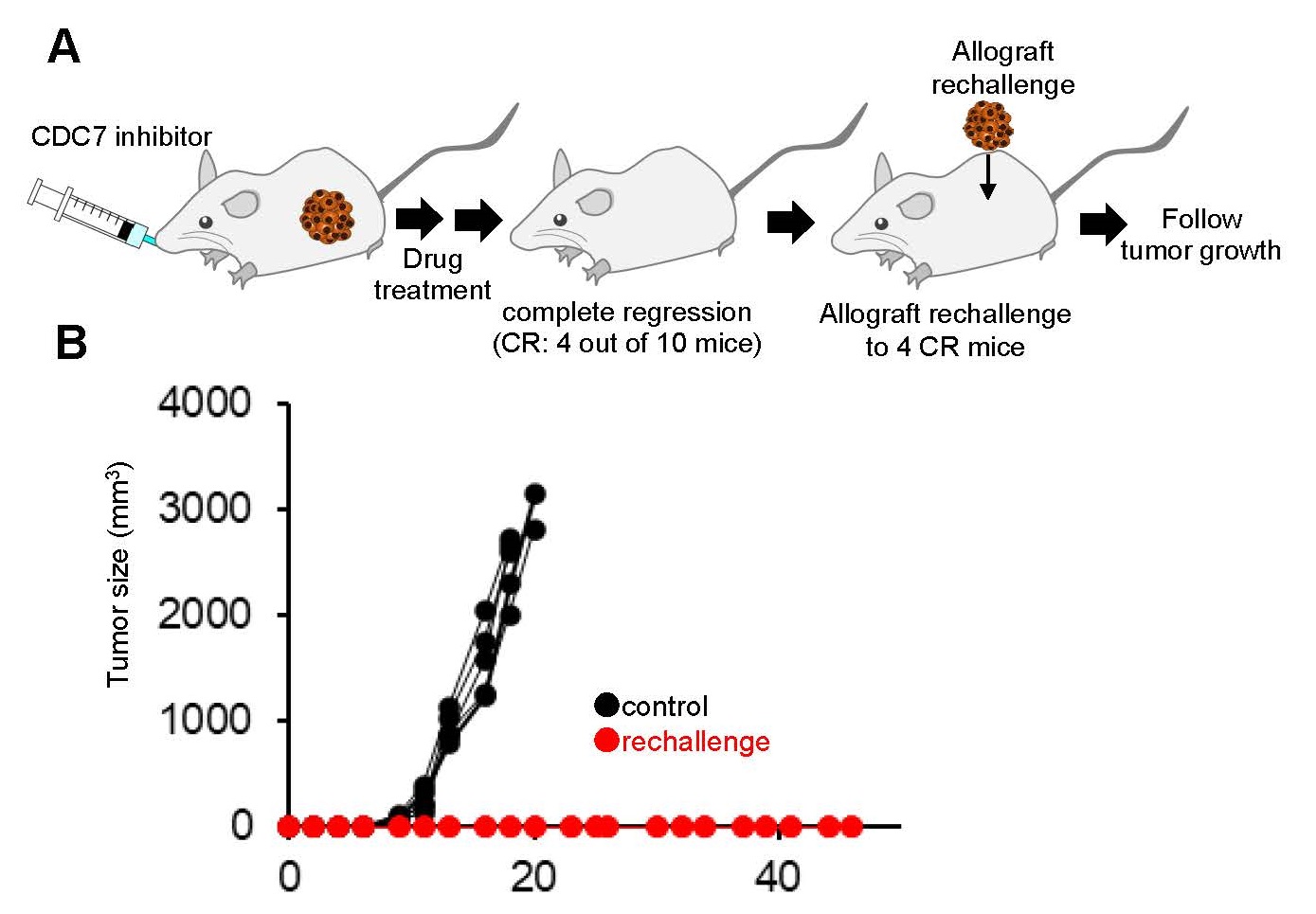
Fig. 3 Retransplantation (rechallenge) experiment using mouse model
A. Re-transplantation (rechallenge) experimental design B. Tumor engraftment data in re-transplanted mice and negative control mice
3. Stress on DNA replication by CDC7 inhibitors enhances the effect of immune checkpoint inhibitors
To investigate the combined effects of CDC7 inhibitors and immune checkpoint inhibitors, we conducted a preclinical efficacy study in combination using mouse subcutaneously allografted tumors. Mice with subcutaneously-allografted mouse tumors were treated with a negative control, the CDC7 inhibitor TAK-931 alone, an anti-mouse PD-1 antibody alone, or a combination with TAK-931 and an anti-mouse PD-1 antibody, and then tumor size was measured in each treatment group (Figure 4A). As Figure 4B shows, the combination with the CDC7 inhibitor TAK-931 and anti-mouse PD-1 antibody caused stronger anti-tumor effects than either alone. These preclinical results suggest that CDC7 inhibitor-mediated DNA replication stress and chromosomal instability appeared to activate an anti-tumor immunity and thus enhance sensitivity to immune checkpoint inhibitors.
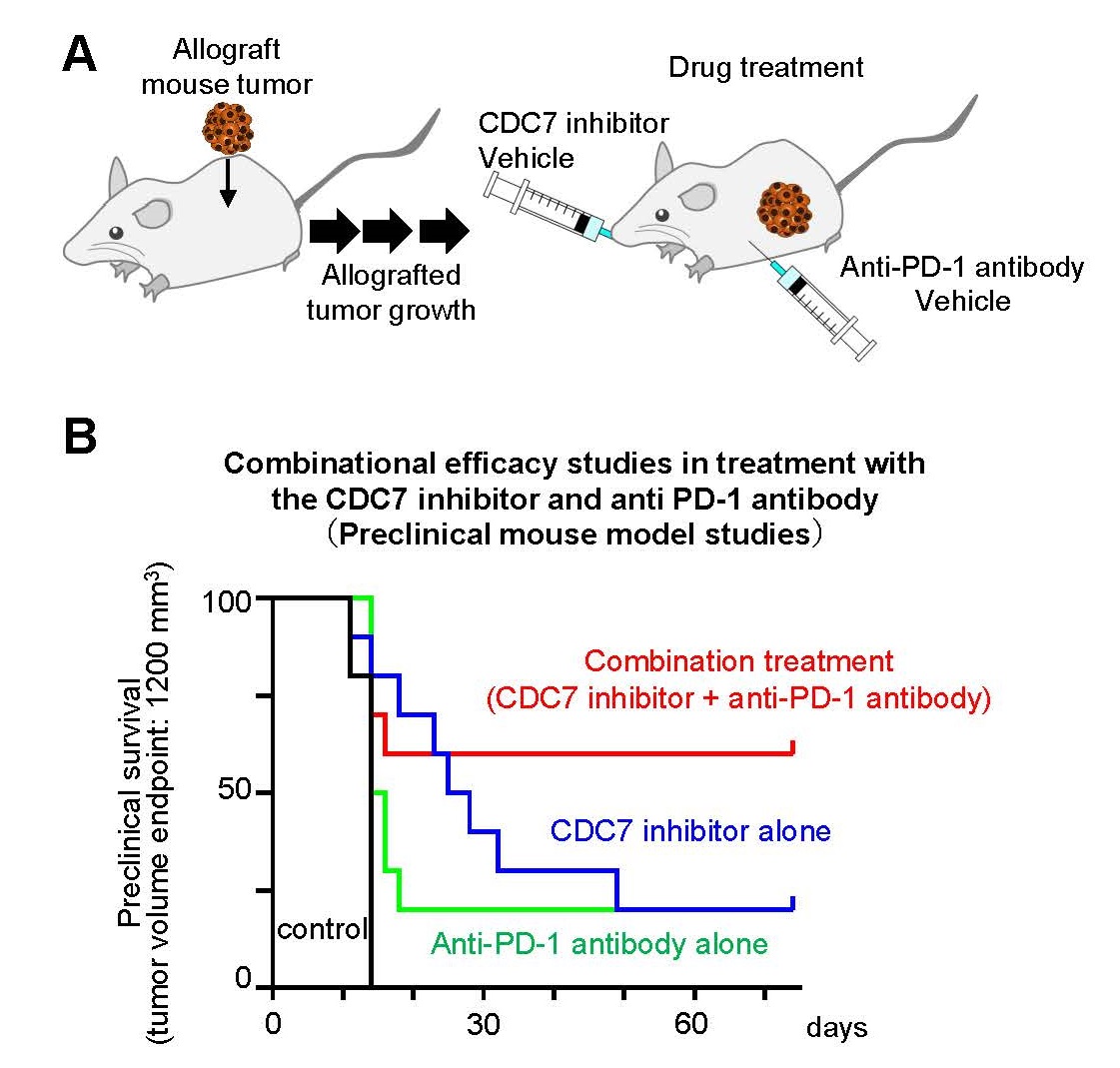
Fig. 4 Preclinical Combination Drug Efficacy Study with ICI in a Mouse Model
A. Experimental design of preclinical combination drug efficacy study with ICI B. Summary of preclinical drug efficacy data for negative control, CDC7 inhibitor alone, mouse ICI (anti-mouse PD-1 antibody) alone and in combination
Outlook
Although many new drugs have been developed along with advances in science and technology, there are still many cancers refractory against existing cancer therapeutic drugs. To overcome such refractory cancers, it is desirable to develop innovative new drugs and therapies with new mechanisms of action, which are different from those of existing cancer drugs. These findings suggest novel cancer immunotherapy targeting DNA replication stress.
Publication
Journal
Nature communications
Title
CDC7 inhibition induces replication stress-mediated aneuploid cells with an inflammatory phenotype sensitizing tumors to immune checkpoint blockade.
Authors
Tomoko Yamamori Morita1†, Jie Yu2†, Yukie Kashima1,3†, Ryo Kamata1, Gaku Yamamoto1, Tatsunori Minamide1,4, Chiaki Mashima1, Miyuki Yoshiya1, Yuta Sakae1, Toyohiro Yamauchi1,5, Yumi Hakozaki1, Shun-ichiro Kageyama6, Akito Nakamura2, Eric Lightcap2, Kosuke Tanaka1, Huifeng Niu7, Karuppiah Kannan8, and Akihiro Ohashi1,2,5,*
- Division of Translational Genomics, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center
- Oncology Drug Discovery Unit, Takeda Development Center Americas (TDCA), Inc.
- Department of Computational Biology and Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo
- Department of Gastroenterology and Endoscopy, National Cancer Center Hospital East
- Department of Integrated Bioscience, Graduate School of Frontier Sciences, The University of Tokyo
- Department of Radiation Oncology, National Cancer Center Hospital East
- Oncology Translational Science., TDCA, Inc.
- Oncology Therapeutic Area Unit, TDCA, Inc.
†These authors contributed equally to this work.
*To whom correspondence should be addressed:
DOI
10.1038/s41467-023-43274-3
Date
November 18,2023
URL
https://doi.org/10.1038/s41467-023-43274-3
Glossary
*1 DNA replication stress (RS)
A condition in which DNA replication is not properly carried out due to drug treatment or other factors.
*2 TAK-931
A small molecule compound that selectively inhibits the enzymatic activity of CDC7 kinase.
*3 Immune checkpoint inhibitor (ICI)
A drug that inhibits the "immune checkpoint," which suppresses T cell activity. Anti-PD-1 antibodies, anti-PD-L1 antibodies, and anti-CTLA4 antibodies are approved therapies.
*4 Chromosome instability
A high frequency of abnormal chromosome segregation, resulting in abnormal chromosome number and structure.
*5 Cytokines and chemokines
Physiologically active substances secreted by cells. Many molecules related to immunity and inflammation induce various cellular responses in target cells. Chemotactic cytokines are especially called chemokines.
Inquiries
On Research
Akihiro Ohashi
Head of Division of Collaborative Research and Development,
Exploratory Oncology Research and Clinical Center
E-mail: aohashi●east.ncc.go.jp
Media Inquiries
Office of Public Relations, Strategic Planning Bureau,
National Cancer Center (Kashiwa Campus)
E-mail: ncc-admin●ncc.go.jp
