Home > Clinical depts > Department of Medical Oncology
Department of Medical Oncology
Toru Mukohara, Ako Hosono, Yoichi Naito, Nobuaki Matsubara, Hirofumi Mukai, Kenichi Harano, Chihiro Kondoh, Takahiro Kogawa, Yoko Fukasawa, Chikako Funasaka, Takeriro Nakao, Shota Kusuhara, Hiromichi Nakajima, Yoriko Hasegawa, Yumi Fujimoto
Introduction
In the clinic, we care for patients with breast, urologic, and gynecologic cancers, as well as sarcoma. We also provide the best available treatment to patients with cancers of unknown primary, multiple primary cancers, or other rare cancers that are difficult to treat in other hospitals.
In 2021, we saw the greatest number of new patients in our department’s history. We newly formed a multidisciplinary conference for bone and soft tissue sarcoma in collaboration with the Department of Musculoskeletal Oncology. We also played a central role in implementing multi-gene panel testing, which came under insurance coverage in 2019. Our department’s chief, Dr. Mukohara, serves as Head of the expert panel (molecular tumor board). We managed 173 out of 383 total cases that were discussed in our expert panel, which contributed to genomic medicine in several regions. We also contributed to the operation of the “Lady’s Center” which opened in 2018 as a core of multi-disciplinary care for women with cancer.
This year, we launched a new investigator-initiated trial (IIT) of limb-cooling as a means to mitigate paclitaxel-induced sensory neuropathy (CECILIA trial) (PI: Toru Mukohara). For another IIT of olaparib +/- pembrolizumab as neoadjuvant therapy for advanced ovarian cancer (PI: Kenichi Harano), which we launched last year, patient recruitment went as planned, and adjunct translational research utilizing single-cell RNA sequence technology is underway in collaboration with the Division of Genome TR. We are also conducting collaborative studies with a technology company to explore the clinical utility of their system for detecting circulating tumor cells (CTCs)
Research activities
We are participating in many company-sponsored trials and other collaborative studies of clinical trial groups, such as JCOG, JBCRG, and WJOG.
We are also implementing clinical and translational research projects with funds obtained from the Grants-in-Aid for Scientific Research (“3D coculture of cancer cells in malignant effusion/CTC with adipose stem cell”; PI: Mukohara T) (“New sub-classification of triple-negative breast cancer to evaluate immunogenicity”; PI: Kogawa T).
In addition, our residents published four English papers under the guidance of the staff members of our department.
Education
Our goal in education is to foster “genuine” medical oncologists. Graduates from our resident / chief resident programs are expected to provide not only standard therapies regardless of cancer type but also multi-disciplinary care in cooperation with other medical professionals. Obtaining skills for palliative care and dealing with oncological emergencies are also expected.
Further, we require and help them to implement clinical research to address clinical questions they develop themselves and report the results in international journals. This is because we aim to develop scientific clinicians who can focus on issues scientifically and create evidence for themselves. As an achievement, this year, two of our residents earned the title Diplomate, Subspecialty Board of Medical Oncology, JSMO.
Further, we earned an educational grant with the theme “Comprehensive educational program to develop medical professionals and peer supporters to empower Adolescent and Young Adult (AYA) breast cancer patients”. We held a multidisciplinary AYA conference (mAYAcon) to deliver optimal care to AYA cancer patients and to conduct educational programs for medical professionals.
Future prospects
In the clinic, we will provide clinical care with high patient satisfaction through a multidisciplinary team approach. In education, we will foster medical oncologists with skills and knowledge for cross-organ oncology care and scientific acumen. In research, we will initiate multi-center clinical trials and Phase I to III developmental trials of investigational drugs. We will also investigate resistance to anti-cancer drugs. Multiple research themes are currently under discussion with industries and other academic organizations.
Table 1. New patient list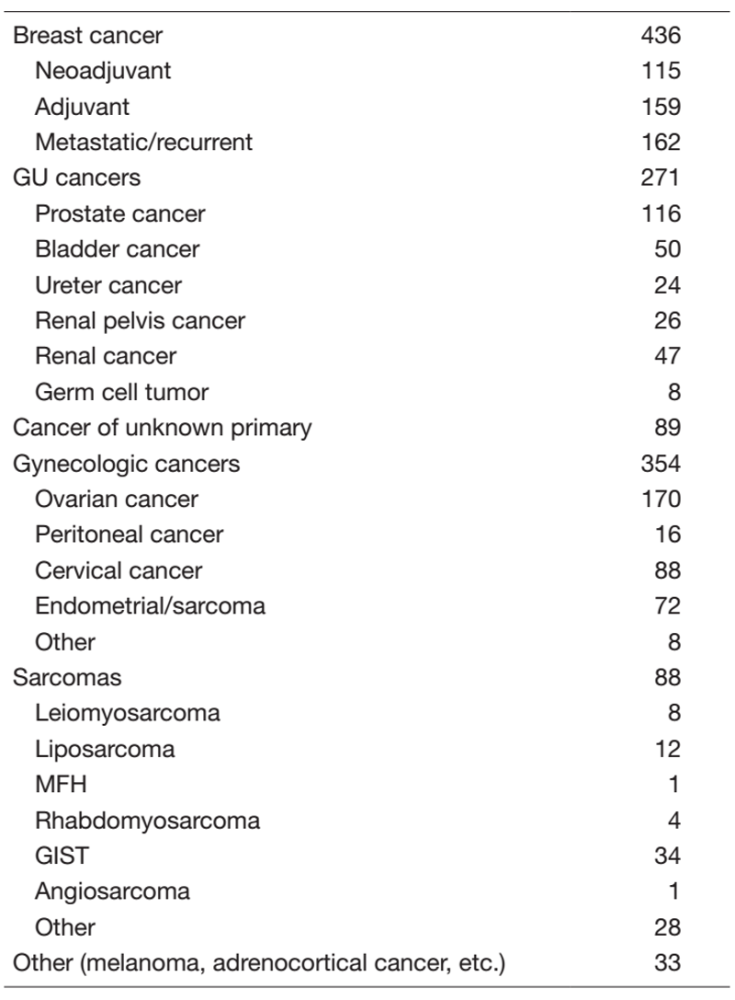
List of papers published
Journal
1. Ohashi Y, Ikeda M, Kunitoh H, Sasako M, Okusaka T, Mukai H, Fujiwara K, Nakamura M, Oba MS, Kimura T, Ibusuki K, Takita A, Sakon M. One-year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: Results from the Cancer-VTE Registry. Thrombosis research, 213:203-213, 2022
2. Ozeki R, Iihara H, Shimokawa M, Hashimoto H, Abe M, Mukohara T, Bando H, Hayashi T, Kawazoe H, Komoda M, Yanai Takahashi T, Saito M. Study protocol for a double-blind, comparative, randomised Japanese trial of triplet standard antiemetic therapies with or without 5 mg olanzapine to prevent chemotherapy-induced nausea and vomiting for patients with breast cancer treated with an anthracycline/cyclophosphamide regimen (JTOP-B). BMJ open, 12:e058755, 2022
3. Thiery-Vuillemin A, de Bono J, Hussain M, Roubaud G, Procopio G, Shore N, Fizazi K, Dos Anjos G, Gravis G, Joung JY, Matsubara N, Castellano D, Degboe A, Gresty C, Kang J, Allen A, Poehlein C, Saad F. Pain and health-related quality of life with olaparib versus physician's choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations (PROfound): an open-label, randomised, phase 3 trial. The Lancet. Oncology, 23:393-405, 2022
4. Doi T, Kuboki Y, Naito Y, Ishida M, Tanaka T, Takeuchi Y. A phase 1 trial of xentuzumab, an IGF-neutralizing antibody, in Japanese patients with advanced solid tumors. Cancer science, 113:1010-1017, 2022
5. Yano M, Nasu K, Yasuda M, Katoh T, Kagabu M, Kobara H, Matsuura M, Tokuyama O, Yamawaki T, Wakahashi S, Noguchi T, Mizuno K, Shitsukawa K, Onohara Y, Nakabori T, Miyasaka A, Nakao T, Matsunaga T, Kunimi Y, Sakurai M, Uchiyama A, Itoh R, Ohike N, Hirakawa T, Watanabe T, Nishino K, Motohashi T, Ito K. Clinicopathological features and programmed death-ligand 1 immunohistochemical expression in a multicenter cohort of uterine and ovarian melanomas: a retrospective study in Japan (KCOG-G1701s). Melanoma research, 32:150-158, 2022
6. Masuda H, Harano K, Miura S, Wang Y, Hirota Y, Harada O, Jolly MK, Matsunaga Y, Lim B, Wood AL, Parinyanitikul N, Jin Lee H, Gong G, George JT, Levine H, Lee J, Wang X, Lucci A, Rao A, Schweitzer BL, Lawrence OR, Seitz RS, Morris SW, Hout DR, Nakamura S, Krishnamurthy S, Ueno NT. Changes in Triple-Negative Breast Cancer Molecular Subtypes in Patients Without Pathologic Complete Response After Neoadjuvant Systemic Chemotherapy. JCO precision oncology, 6:e2000368, 2022
7. Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B, Montesa-Pino á, Ye D, Parnis F, Cruz F, Tammela TLJ, Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H, Thiele S, Li R, Kuss I, Tombal B . Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. The New England journal of medicine, 386:1132-1142, 2022
8. Matsubara N, Nishimura K, Kawakami S, Joung JY, Uemura H, Goto T, Kwon TG, Sugimoto M, Kato M, Wang SS, Pang ST, Chen CH, Fujita T, Nii M, Shen L, Dujka M, Hussain M, de Bono J. Olaparib in patients with mCRPC with homologous recombination repair gene alterations: PROfound Asian subset analysis. Japanese journal of clinical oncology, 52:441-448, 2022
9. Nakamura Y, Okamoto W, Denda T, Nishina T, Komatsu Y, Yuki S, Yasui H, Esaki T, Sunakawa Y, Ueno M, Shinozaki E, Matsuhashi N, Ohta T, Kato K, Ohtsubo K, Bando H, Hara H, Satoh T, Yamazaki K, Yamamoto Y, Okano N, Terazawa T, Kato T, Oki E, Tsuji A, Horita Y, Hamamoto Y, Kawazoe A, Nakajima H, Nomura S, Mitani R, Yuasa M, Akagi K, Yoshino T. Clinical Validity of Plasma-Based Genotyping for Microsatellite Instability Assessment in Advanced GI Cancers: SCRUM-Japan GOZILA Substudy. JCO precision oncology, 6:e2100383, 2022
10. Nakajima H, Harano K, Nakai T, Kusuhara S, Nakao T, Funasaka C, Kondoh C, Matsubara N, Naito Y, Hosono A, Mitsunaga S, Ishii G, Mukohara T. Impacts of clinicopathological factors on efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer. Breast (Edinburgh, Scotland), 61:136-144, 2022
11. Baciarello G, Özgüroğlu M, Mundle S, Leitz G, Richarz U, Hu P, Feyerabend S, Matsubara N, Chi KN, Fizazi K . Impact of abiraterone acetate plus prednisone in patients with castration-sensitive prostate cancer and visceral metastases over four years of follow-up: A post-hoc exploratory analysis of the LATITUDE study. European journal of cancer (Oxford, England: 1990), 162:56-64, 2022
12. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Untch M, Fasching PA, Cardoso F, Andersen J, Patt D, Danso M, Ferreira M, Mouret-Reynier MA, Im SA, Ahn JH, Gion M, Baron-Hay S, Boileau JF, Ding Y, Tryfonidis K, Aktan G, Karantza V, O’Shaughnessy J . Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. The New England journal of medicine, 386:556-567, 2022
13. Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F. Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast cancer (Tokyo, Japan), 29:131-143, 2022
14. Taira N, Kashiwabara K, Tsurutani J, Kitada M, Takahashi M, Kato H, Kikawa Y, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Takashima T, Aihara T, Mukai H, Hara F. Correction to: Quality of life in a randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast cancer (Tokyo, Japan), 29:186-188, 2022
15. Negishi R, Yamakawa H, Kobayashi T, Horikawa M, Shimoyama T, Koizumi F, Sawada T, Oboki K, Omuro Y, Funasaka C, Kageyama A, Kanemasa Y, Tanaka T, Matsunaga T, Yoshino T. Transcriptomic profiling of single circulating tumor cells provides insight into human metastatic gastric cancer. Communications biology, 5:20, 2022
16. Koroki Y, Taguri M, Matsubara N, Fizazi K. Estimation of Overall Survival with Subsequent Treatment Effect by Applying Inverse Probability of Censoring Weighting in the LATITUDE Study. European urology open science, 36:51-58, 2022
17. de Wit R, Powles T, Castellano D, Necchi A, Lee JL, van der Heijden MS, Matsubara N, Bamias A, Fléchon A, Sternberg CN, Drakaki A, Yu EY, Zimmermann AH, Long A, Walgren RA, Gao L, Bell-McGuinn KM, Petrylak DP . Exposure-response relationship of ramucirumab in RANGE, a randomized phase III trial in advanced urothelial carcinoma refractory to platinum therapy. British journal of clinical pharmacology, 2022
18. Kawahara T, Taira N, Shiroiwa T, Hagiwara Y, Fukuda T, Uemura Y, Mukai H. Minimal important differences of EORTC QLQ-C30 for metastatic breast cancer patients: Results from a randomized clinical trial. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation, 31:1829-1836, 2022
19. Hussain M, Corcoran C, Sibilla C, Fizazi K, Saad F, Shore N, Sandhu S, Mateo J, Olmos D, Mehra N, Kolinsky MP, Roubaud G, Özgüroǧlu M, Matsubara N, Gedye C, Choi YD, Padua C, Kohlmann A, Huisden R, Elvin JA, Kang J, Adelman CA, Allen A, Poehlein C, de Bono J . Tumor Genomic Testing for >4,000 Men with Metastatic Castration-resistant Prostate Cancer in the Phase III Trial PROfound (Olaparib). Clinical cancer research: an official journal of the American Association for Cancer Research, 28:1518-1530, 2022
20. Kunisada T, Nakata E, Fujiwara T, Hosono A, Takihira S, Kondo H, Ozaki T. Soft-tissue sarcoma in adolescents and young adults. International journal of clinical oncology, 2022
21. Tamada S, Kondoh C, Matsubara N, Mizuno R, Kimura G, Anai S, Tomita Y, Oyama M, Masumori N, Kojima T, Matsumoto H, Chen M, Li M, Matsuda K, Tanaka Y, Rini BI, Uemura H. Pembrolizumab plus axitinib versus sunitinib in metastatic renal cell carcinoma: outcomes of Japanese patients enrolled in the randomized, phase III, open-label KEYNOTE-426 study. International journal of clinical oncology, 27:154-164, 2022
22. Powles T, Yuen KC, Gillessen S, Kadel EE 3rd, Rathkopf D, Matsubara N, Drake CG, Fizazi K, Piulats JM, Wysocki PJ, Buchschacher GL Jr, Alekseev B, Mellado B, Karaszewska B, Doss JF, Rasuo G, Datye A, Mariathasan S, Williams P, Sweeney CJ . Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nature medicine, 28:144-153, 2022
23. Rini BI, Atkins MB, Plimack ER, Soulières D, McDermott RS, Bedke J, Tartas S, Alekseev B, Melichar B, Shparyk Y, Kondoh C, Langiewicz P, Wood LA, Hammers H, Silber CG, Haber B, Jensen E, Chen M, Powles T . Characterization and Management of Treatment-emergent Hepatic Toxicity in Patients with Advanced Renal Cell Carcinoma Receiving First-line Pembrolizumab plus Axitinib. Results from the KEYNOTE-426 Trial. European urology oncology, 5:225-234, 2022
24. Tan RSYC, Ong WS, Lee KH, Lim AH, Park S, Park YH, Lin CH, Lu YS, Ono M, Ueno T, Naito Y, Onishi T, Lim GH, Tan SM, Lee HB, Ryu HS, Han W, Tan VKM, Wong FY, Im SA, Tan PH, Chan JY, Yap YS. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC medicine, 20:105, 2022
25. Kawai A, Naka N, Shimomura A, Takahashi S, Kitano S, Imura Y, Yonemori K, Nakatani F, Iwata S, Kobayashi E, Outani H, Tamiya H, Naito Y, Yamamoto N, Doi T. Efficacy and safety of TAS-115, a novel oral multi-kinase inhibitor, in osteosarcoma: an expansion cohort of a phase I study. Investigational new drugs, 39:1559-1567, 2021
26. Sunami K, Bando H, Yatabe Y, Naito Y, Takahashi H, Tsuchihara K, Toyooka S, Mimori K, Kohsaka S, Uetake H, Kinoshita I, Komine K, Takeda M, Hayashida T, Tamura K, Nishio K, Yamamoto N. Appropriate use of cancer comprehensive genome profiling assay using circulating tumor DNA. Cancer science, 112:3911-3917, 2021
27. Doi T, Yamamoto N, Naito Y, Kuboki Y, Koyama T, Piao Y, Tsujimoto N, Asou H, Inoue K, Kondo S. Merestinib monotherapy or in combination for japanese patients with advanced and/or metastatic cancer: A phase 1 study. Cancer medicine, 10:6579-6589, 2021
28. Shimoi T, Nagai SE, Yoshinami T, Takahashi M, Arioka H, Ishihara M, Kikawa Y, Koizumi K, Kondo N, Sagara Y, Takada M, Takano T, Tsurutani J, Naito Y, Nakamura R, Hattori M, Hara F, Hayashi N, Mizuno T, Miyashita M, Yamashita N, Yamanaka T, Saji S, Iwata H, Toyama T. Correction to: The Japanese Breast Cancer Society Clinical Practice Guidelines for systemic treatment of breast cancer, 2018 edition. Breast cancer (Tokyo, Japan), 28:985-986, 2021
29. Mamishin K, Naito Y, Nomura S, Ogawa G, Niguma K, Baba K, Sakaeda S, Nakajima H, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. Comparison of Treatment Completion Rate Between Conventional and Dose-dense Doxorubicin and Cyclophosphamide (AC) Followed by a Taxane in Patients With Breast Cancer: A Propensity Score-matched Analysis. Anticancer research, 41:6217-6224, 2021
30. Naito Y. [II. Application for HER2-Positive Breast Cancer(Trastuzumab Deruxtecan)]. Gan to kagaku ryoho. Cancer & chemotherapy, 48:1452-1453, 2021
31. Sakaeda S, Naito Y. Circulating Tumor DNA in Oncology. Processes, 9:2198, 2021
32. El Bairi K, Haynes HR, Blackley E, Fineberg S, Shear J, Turner S, de Freitas JR, Sur D, Amendola LC, Gharib M, Kallala A, Arun I, Azmoudeh-Ardalan F, Fujimoto L, Sua LF, Liu SW, Lien HC, Kirtani P, Balancin M, El Attar H, Guleria P, Yang W, Shash E, Chen IC, Bautista V, Do Prado Moura JF, Rapoport BL, Castaneda C, Spengler E, Acosta-Haab G, Frahm I, Sanchez J, Castillo M, Bouchmaa N, Md Zin RR, Shui R, Onyuma T, Yang W, Husain Z, Willard-Gallo K, Coosemans A, Perez EA, Provenzano E, Ericsson PG, Richardet E, Mehrotra R, Sarancone S, Ehinger A, Rimm DL, Bartlett JMS, Viale G, Denkert C, Hida AI, Sotiriou C, Loibl S, Hewitt SM, Badve S, Symmans WF, Kim RS, Pruneri G, Goel S, Francis PA, Inurrigarro G, Yamaguchi R, Garcia-Rivello H, Horlings H, Afqir S, Salgado R, Adams S, Kok M, Dieci MV, Michiels S, Demaria S, Loi S. The tale of TILs in breast cancer: A report from The International Immuno-Oncology Biomarker Working Group. NPJ breast cancer, 7:150, 2021
33. Naito Y, Kuboki Y, Ikeda M, Harano K, Matsubara N, Toyoizumi S, Mori Y, Hori N, Nagasawa T, Kogawa T. Safety, pharmacokinetics, and preliminary efficacy of the PARP inhibitor talazoparib in Japanese patients with advanced solid tumors: phase 1 study. Investigational new drugs, 39:1568-1576, 2021
34. Kawazoe A, Itahashi K, Yamamoto N, Kotani D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M, Higuchi T, Ikeno T, Wakabayashi M, Sato A, Koyama S, Nishikawa H, Shitara K. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clinical cancer research: an official journal of the American Association for Cancer Research, 27:6709-6715, 2021
35. Matsubara N, Uemura H, Nagamori S, Suzuki H, Uemura H, Kimura G. A Phase II, Randomized, Open-Label, Multi-arm Study of TAS-115 for Castration-Resistant Prostate Cancer Patients With Bone Metastases. Clinical genitourinary cancer, 19:491-500, 2021
36. Uemura M, Nakaigawa N, Sassa N, Tatsugami K, Harada K, Yamasaki T, Matsubara N, Yoshimoto T, Nakagawa Y, Fukuyama T, Oya M, Shinohara N, Uemura H, Tsuzuki T. Prognostic value of programmed death-ligand 1 status in Japanese patients with renal cell carcinoma. International journal of clinical oncology, 26:2073-2084, 2021
37. Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E, Yañez E, Gümüş M, Olivera Hurtado de Mendoza M, Samouëlian V, Castonguay V, Arkhipov A, Toker S, Li K, Keefe SM, Monk BJ . Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. The New England journal of medicine, 385:1856-1867, 2021
38. Uemura M, Nakaigawa N, Sassa N, Tatsugami K, Harada K, Yamasaki T, Matsubara N, Yoshimoto T, Nakagawa Y, Fukuyama T, Oya M, Shinohara N, Uemura H, Tsuzuki T. Correction to: Prognostic value of programmed death-ligand 1 status in Japanese patients with renal cell carcinoma. International journal of clinical oncology, 26:2085-2086, 2021
39. Akimoto E, Tokunaga M, Sato R, Yoshida A, Naito Y, Yamashita R, Kinoshita T, Kuwata T. Gastric mesenchymal tumor with smooth muscle differentiation and echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (EML4-ALK) fusion. Pathology international, 71:707-711, 2021
40. Mukai H, Uemura Y, Akabane H, Watanabe T, Park Y, Takahashi M, Sagara Y, Nishimura R, Takashima T, Fujisawa T, Hozumi Y, Kawahara T. Anthracycline-containing regimens or taxane versus S-1 as first-line chemotherapy for metastatic breast cancer. British journal of cancer, 125:1217-1225, 2021
41. Takashima T, Hara F, Iwamoto T, Uemura Y, Ohsumi S, Yotsumoto D, Hozumi Y, Watanabe T, Saito T, Watanabe KI, Tsurutani J, Toyama T, Akabane H, Nishimura R, Taira N, Ohashi Y, Mukai H. A Correlation Analysis Between Metabolism-related Genes and Treatment Response to S-1 as First-line Chemotherapy for Metastatic Breast Cancer: The SELECT BC-EURECA Study. Clinical breast cancer, 21:450-457, 2021
42. Inoue K, Masuda N, Iwata H, Takahashi M, Ito Y, Miyoshi Y, Nakayama T, Mukai H, van der Walt JS, Mori J, Sakaguchi S, Kawaguchi T, Tanizawa Y, Llombart-Cussac A, Sledge GW Jr, Toi M. Japanese subpopulation analysis of MONARCH 2: phase 3 study of abemaciclib plus fulvestrant for treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer that progressed on endocrine therapy. Breast cancer (Tokyo, Japan), 28:1038-1050, 2021
43. Funasaka C, Naito Y, Kusuhara S, Nakao T, Fukasawa Y, Mamishin K, Komuro A, Okunaka M, Kondoh C, Harano K, Kogawa T, Matsubara N, Hosono A, Kawasaki T, Mukohara T. The efficacy and safety of paclitaxel plus bevacizumab therapy in breast cancer patients with visceral crisis. Breast (Edinburgh, Scotland), 58:50-56, 2021
44. Aoki H, Ueha S, Nakamura Y, Shichino S, Nakajima H, Shimomura M, Sato A, Nakatsura T, Yoshino T, Matsushima K. Greater extent of blood-tumor TCR repertoire overlap is associated with favorable clinical responses to PD-1 blockade. Cancer science, 112:2993-3004, 2021
45. Early Breast Cancer Trialists’ Collaborative group (EBCTCG). Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13?864 women in seven randomised trials. The Lancet. Oncology, 22:1139-1150, 2021
46. Hagiwara Y, Sawaki M, Uemura Y, Kawahara T, Shimozuma K, Ohashi Y, Takahashi M, Saito T, Baba S, Kobayashi K, Mukai H, Taira N. Impact of chemotherapy on cognitive functioning in older patients with HER2-positive breast cancer: a sub-study in the RESPECT trial. Breast cancer research and treatment, 188:675-683, 2021
47. Taira N, Sawaki M, Uemura Y, Saito T, Baba S, Kobayashi K, Kawashima H, Tsuneizumi M, Sagawa N, Bando H, Takahashi M, Yamaguchi M, Takashima T, Nakayama T, Kashiwaba M, Mizuno T, Yamamoto Y, Iwata H, Ohashi Y, Mukai H, Kawahara T. Health-Related Quality of Life With Trastuzumab Monotherapy Versus Trastuzumab Plus Standard Chemotherapy as Adjuvant Therapy in Older Patients With HER2-Positive Breast Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 39:2452-2462, 2021
48. Mizumoto M, Fuji H, Miyachi M, Soejima T, Yamamoto T, Aibe N, Demizu Y, Iwata H, Hashimoto T, Motegi A, Kawamura A, Terashima K, Fukushima T, Nakao T, Takada A, Sumi M, Oshima J, Moriwaki K, Nozaki M, Ishida Y, Kosaka Y, Ae K, Hosono A, Harada H, Ogo E, Akimoto T, Saito T, Fukushima H, Suzuki R, Takahashi M, Matsuo T, Matsumura A, Masaki H, Hosoi H, Shigematsu N, Sakurai H. Proton beam therapy for children and adolescents and young adults (AYAs): JASTRO and JSPHO Guidelines. Cancer treatment reviews, 98:102209, 2021
49. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Fléchon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A . Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. The Lancet. Oncology, 22:931-945, 2021
50. Sweeney C, Bracarda S, Sternberg CN, Chi KN, Olmos D, Sandhu S, Massard C, Matsubara N, Alekseev B, Parnis F, Atduev V, Buchschacher GL Jr, Gafanov R, Corrales L, Borre M, Stroyakovskiy D, Alves GV, Bournakis E, Puente J, Harle-Yge ML, Gallo J, Chen G, Hanover J, Wongchenko MJ, Garcia J, de Bono JS. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet (London, England), 398:131-142, 2021
51. Takahashi M, Inoue K, Mukai H, Yamanaka T, Egawa C, Miyoshi Y, Sakata Y, Muramoto K, Ikezawa H, Matsuoka T, Tsurutani J. Indices of peripheral leukocytes predict longer overall survival in breast cancer patients on eribulin in Japan. Breast cancer (Tokyo, Japan), 28:945-955, 2021
52. Hagiwara Y, Shinozaki T, Mukai H, Matsuyama Y. Sensitivity analysis for subsequent treatments in confirmatory oncology clinical trials: A two-stage stochastic dynamic treatment regime approach. Biometrics, 77:702-714, 2021
53. Nakajima H, Kotani D, Bando H, Kato T, Oki E, Shinozaki E, Sunakawa Y, Yamazaki K, Yuki S, Nakamura Y, Yamanaka T, Yoshino T, Ohta T, Taniguchi H, Kagawa Y. REMARRY and PURSUIT trials: liquid biopsy-guided rechallenge with anti-epidermal growth factor receptor (EGFR) therapy with panitumumab plus irinotecan for patients with plasma RAS wild-type metastatic colorectal cancer. BMC cancer, 21:674, 2021
54. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A, Balmaña J, Domchek SM, Gelmon KA, Hollingsworth SJ, Korde LA, Linderholm B, Bandos H, Senkus E, Suga JM, Shao Z, Pippas AW, Nowecki Z, Huzarski T, Ganz PA, Lucas PC, Baker N, Loibl S, McConnell R, Piccart M, Schmutzler R, Steger GG, Costantino JP, Arahmani A, Wolmark N, McFadden E, Karantza V, Lakhani SR, Yothers G, Campbell C, Geyer CE Jr . Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. The New England journal of medicine, 384:2394-2405, 2021
55. Nishida T, Yoshinaga S, Takahashi T, Naito Y. Recent Progress and Challenges in the Diagnosis and Treatment of Gastrointestinal Stromal Tumors. Cancers, 13:2021
56. Zhu A, Yuan P, Hu N, Li M, Wang W, Wang X, Yue J, Wang J, Luo Y, Ma F, Zhang P, Li Q, Xu B, Cao S, Lippi G, Naito Y, Osman MA, Marta GN, Franceschini G, Orlandi A. Phase II study of apatinib in combination with oral vinorelbine in heavily pretreated HER2-negative metastatic breast cancer and clinical implications of monitoring ctDNA. Cancer biology & medicine, 18:875-887, 2021
57. Doi T, Tajimi M, Mori J, Asou H, Inoue K, Benhadji KA, Naito Y. A phase 1 study of crenigacestat (LY3039478), the Notch inhibitor, in Japanese patients with advanced solid tumors. Investigational new drugs, 39:469-476, 2021
58. Sagara Y, Mori M, Yamamoto S, Eguchi K, Iwatani T, Naito Y, Kogawa T, Tanaka K, Kotani H, Yasojima H, Ozaki Y, Noguchi E, Miyasita M, Kondo N, Niikura N, Toi M, Shien T, Iwata H. Current Status of Advance Care Planning and End-of-life Communication for Patients with Advanced and Metastatic Breast Cancer. The oncologist, 26:e686-e693, 2021
59. Ishikawa T, Sakamaki K, Narui K, Nishimura H, Sangai T, Tamaki K, Hasegawa Y, Watanabe KI, Suganuma N, Michishita S, Sugae S, Aihara T, Tsugawa K, Kaise H, Taira N, Mukai H. Prospective cohort study of febrile neutropenia in breast cancer patients administered with neoadjuvant and adjuvant chemotherapies: CSPOR-BC FN study. Breast (Edinburgh, Scotland), 56:70-77, 2021
60. Umeda M, Ota Y, Kashiwabara K, Hayashi N, Naito M, Yamashita T, Mukai H, Nakatsukasa K, Amemiya T, Watanabe KI, Hata H, Kikawa Y, Taniike N, Yamanaka T, Mitsunaga S, Nakagami K, Adachi M, Kondo N, Shibuya Y, Niikura N. Oral care and oral assessment guide in breast cancer patients receiving everolimus and exemestane: subanalysis of a randomized controlled trial (Oral Care-BC). Annals of translational medicine, 9:535, 2021
61. Umeda K, Miyamura T, Yamada K, Sano H, Hosono A, Sumi M, Okita H, Kumamoto T, Kawai A, Hirayama J, Jyoko R, Sawada A, Nakayama H, Hosoya Y, Maeda N, Yamamoto N, Imai C, Hasegawa D, Chin M, Ozaki T. Clinical outcome of patients with recurrent or refractory localized Ewing's sarcoma family of tumors: A retrospective report from the Japan Ewing Sarcoma Study Group. Cancer reports (Hoboken, N.J.), 4:e1329, 2021
62. Mukohara T, Hosono A, Mimaki S, Nakayama A, Kusuhara S, Funasaka C, Nakao T, Fukasawa Y, Kondoh C, Harano K, Naito Y, Matsubara N, Tsuchihara K, Kuwata T. Effects of Ado-Trastuzumab Emtansine and Fam-Trastuzumab Deruxtecan on Metastatic Breast Cancer Harboring HER2 Amplification and the L755S Mutation. The oncologist, 26:635-639, 2021
63. Kuroe T, Watanabe R, Kojima M, Morisue R, Sugano M, Kuwata T, Masuda H, Kusuhara S, Matsubara N, Oda S, Ushiku T, Ishii G. Evaluation of the morphological features and unfavorable prognostic impact of dirty necrosis in renal cell carcinoma. Journal of cancer research and clinical oncology, 147:1089-1100, 2021
64. Kijima T, Fukushima H, Kusuhara S, Tanaka H, Yoshida S, Yokoyama M, Ishioka J, Matsuoka Y, Numao N, Sakai Y, Saito K, Matsubara N, Yuasa T, Masuda H, Yonese J, Kageyama Y, Fujii Y. Association Between the Occurrence and Spectrum of Immune-Related Adverse Events and Efficacy of Pembrolizumab in Asian Patients With Advanced Urothelial Cancer: Multicenter Retrospective Analyses and Systematic Literature Review. Clinical genitourinary cancer, 19:208-216.e1, 2021
