Annual Report 2019
Department of Musculoskeletal Oncology and Rehabilitation
Akira Kawai, Fumihiko Nakatani, Eisuke Kobayashi, Shintaro Iwata, Suguru Fukushima, Makoto Nakagawa, Tadashi Komatsubara, Masato Saito, Jun Sugaya, Chiaki Sato, Noriko Watanabe, Takurou Sakurai, Sachiko Yahiro, Mami Oki, Yusuke Okita, Takuya Fukushima, Shouta Yokota, Aiko Matsuoka, Kazuhiro Kojima
Introduction
Malignant tumors arising from connective tissue are extremely rare: they are estimated to account for only 0.01% of newly developed cancers. The rarity itself sometimes causes several problems in treating patients with bone and soft tissue tumors, including retardation of accurate diagnoses and a lack of understanding regarding standardized therapeutic approaches. Since 1962, the Musculoskeletal Oncology Division of the National Cancer Center Hospital (NCCH) has been accumulating a vast array of clinical knowledge regarding musculoskeletal tumors in collaboration with radiologists and pathologists specializing in sarcomas, which has enabled us to offer well-organized treatment strategies to patients with various types of bone and soft tissue tumors. We have also been conducting basic and clinical studies using accumulated clinical samples and information to establish novel diagnostic methods and therapeutic approaches for treating musculoskeletal tumors. In addition, we have given weight to clinical trials on three different but inseparable fields: surgery, chemotherapy and radiation therapy for bone and soft tissue tumors.
The Team and What We Do
The Musculoskeletal Oncology Division of the NCCH consists of six staff doctors, three residents and four physiotherapists, three occupational therapists and two speech therapists. Occasionally, several fellows from Japan and overseas join our group. Outpatient consultations are held every weekday. A constant number of over 25 patients are hospitalized for operations, chemotherapy or radiation therapy. Six to 10 major operations are routinely performed every week. In 2019, as shown in Table 1, 362 operations were performed, including palliative operations for pathological fractures from metastatic bone and soft tissue tumors. Sarcomas in the trunk, including 32 cases of soft tissue sarcoma, 16 cases of bone sarcomas and 17 cases in the retroperitoneal space were excised in cooperation with thoracic, general or urological surgeons. A total of 75 reconstructive operations were conducted in collaboration with plastic surgeons to achieve adequate soft tissue coverage after the resection of malignant tumors of the trunk or limb-salvage operations for sarcomas of the extremities. As a result, almost 90% of the operations were performed with a limb-sparing approach. With regard to the patients’ postoperative course, we have been collaborating with a physical therapist to rehabilitate the musculoskeletal system in cancer-bearing patients.
As for chemotherapy, we have been conducting neo-adjuvant and adjuvant chemotherapy for high-grade bone and soft tissue tumors, palliative chemotherapy for metastatic bone and soft tissue sarcomas, where necessary in collaboration with medical oncologists. We have been collaborating with pediatric oncologists for chemotherapeutic treatment of children and adolescents with sarcomas.
Table 1. Type of surgical procedure (2019)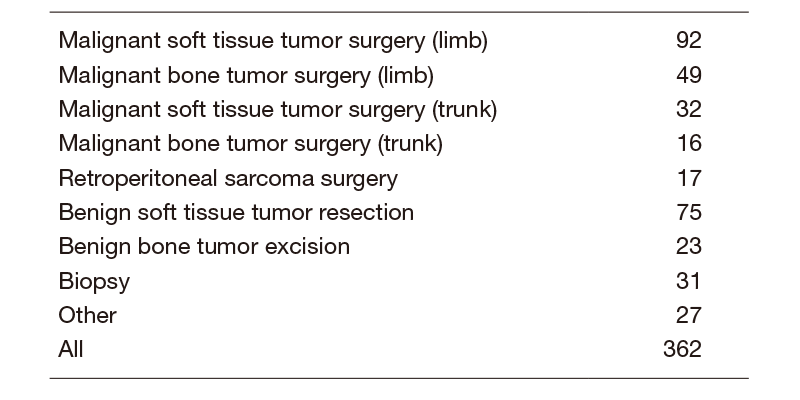

Research activities
Since 2004, we have been collaborating with the NCC Research Institute to develop novel molecular target therapies or tailor-made treatments for sarcoma patients. With a new generation sequence system, we have been analyzing the complete expression levels of genomic alteration in the tumor samples or serum samples from patients with various types of bone and soft tissue sarcoma. Combined with each patient’s clinical information, we have been establishing novel biomarkers for prediction of patients’ prognoses or effects of the chemotherapeutic agents. We have also been developing mouse xenograft models of sarcoma and novel sarcoma cell lines using the clinical samples of biopsied or resected tumors with the aim to create novel molecular targeted therapies or biomarkers.
Clinical trials
1. A multi-institutional phase III clinical trial of multi-drugs adjuvant chemotherapy for osteosarcomas (The Japan Clinical Oncology Group (JCOG) 0905) since 2010.
2. A multi-institutional phase III clinical trial of multi-drugs adjuvant chemotherapy for osteosarcomas (JCOG 1306) since 2014.
3. A multi-institutional phase III clinical trial of preoperative denosumab for giant cell tumor (JCOG 1610) since 2017
4. A multi-institutional randomized phase II clinical trial of second line chemotherapy for advanced soft tissue sarcoma (JCOG 1802) since 2019
Education
Each resident performs 60 to70 operations supervised by staff members every year, joins many domestic and international conferences and publishes several medical articles or reports during training courses.
Future prospects
Our division continues to focus on the development of novel therapeutic tools for the patients with bone and soft tissue tumors collaborating with other clinical divisions and research institutes.
List of papers published in 2019
Journal
1. Doi T, Matsubara N, Kawai A, Naka N, Takahashi S, Uemura H, Yamamoto N. Phase I study of TAS-115, a novel oral multi-kinase inhibitor, in patients with advanced solid tumors. Invest New Drugs, 38:1175-1185, 2020
2. Frezza AM, Assi T, Lo Vullo S, Ben-Ami E, Dufresne A, Yonemori K, Noguchi E, Siontis B, Ferraro R, Teterycz P, Duffaud F, Ravi V, Vincenzi B, Gelderblom H, Pantaleo MA, Baldi GG, Desar I, Fedenko A, Maki RG, Jones RL, Benjamin RS, Blay JY, Kawai A, Gounder M, Gronchi A, Le Cesne A, Mir O, Czarnecka AM, Schuetze S, Wagner AJ, Adam J, Barisella M, Sbaraglia M, Hornick JL, Meurgey A, Mariani L, Casali PG, Thornton K, Stacchiotti S. Systemic treatments in MDM2 positive intimal sarcoma: A multicentre experience with anthracycline, gemcitabine, and pazopanib within the World Sarcoma Network. Cancer, 126:98-104, 2020
3. Okuma HS, Yonemori K, Narita SN, Sukigara T, Hirakawa A, Shimizu T, Shibata T, Kawai A, Yamamoto N, Nakamura K, Nishida T, Fujiwara Y. MASTER KEY Project: Powering Clinical Development for Rare Cancers Through a Platform Trial. Clin Pharmacol Ther, 2020
4. Kobayashi H, Iwata S, Wakamatsu T, Hayakawa K, Yonemoto T, Wasa J, Oka H, Ueda T, Tanaka S. Efficacy and safety of trabectedin for patients with unresectable and relapsed soft-tissue sarcoma in Japan: A Japanese Musculoskeletal Oncology Group study. Cancer, 126:1253-1263, 2020
5. Ogura K, Uehara K, Akiyama T, Shinoda Y, Iwata S, Tsukushi S, Kobayashi E, Hirose T, Yonemoto T, Endo M, Tanzawa Y, Nakatani F, Kawano H, Tanaka S, Kawai A. Minimal clinically important differences in Toronto Extremity Salvage Score for patients with lower extremity sarcoma. J Orthop Sci, 25:315-318, 2020
6. Oyama R, Kito F, Takahashi M, Hattori E, Noguchi R, Takai Y, Sakumoto M, Qiao Z, Toki S, Sugawara M, Tanzawa Y, Kobayashi E, Nakatani F, Iwata S, Yoshida A, Kawai A, Kondo T. Establishment and characterization of patient-derived cancer models of malignant peripheral nerve sheath tumors. Cancer Cell Int, 20:58, 2020
7. Machida Y, Nakagawa M, Matsunaga H, Yamaguchi M, Ogawara Y, Shima Y, Yamagata K, Katsumoto T, Hattori A, Itoh M, Seki T, Nishiya Y, Nakamura K, Suzuki K, Imaoka T, Baba D, Suzuki M, Sampetrean O, Saya H, Ichimura K, Kitabayashi I. A Potent Blood-Brain Barrier-Permeable Mutant IDH1 Inhibitor Suppresses the Growth of Glioblastoma with IDH1 Mutation in a Patient-Derived Orthotopic Xenograft Model. Mol Cancer Ther, 19:375-383, 2020
8. Yahiro K, Matsumoto Y, Yamada H, Endo M, Setsu N, Fujiwara T, Nakagawa M, Kimura A, Shimada E, Okada S, Oda Y, Nakashima Y. Activation of TLR4 signaling inhibits progression of osteosarcoma by stimulating CD8-positive cytotoxic lymphocytes. Cancer Immunol Immunother, 69:745-758, 2020
9. Yoshimatsu Y, Noguchi R, Tsuchiya R, Kito F, Sei A, Sugaya J, Nakagawa M, Yoshida A, Iwata S, Kawai A, Kondo T. Establishment and characterization of NCC-CDS2-C1: a novel patient-derived cell line of CIC-DUX4 sarcoma. Hum Cell, 33:427-436, 2020
10. Heng M, Gupta A, Chung PW, Healey JH, Vaynrub M, Rose PS, Houdek MT, Lin PP, Bishop AJ, Hornicek FJ, Chen YL, Lozano-Calderon S, Holt GE, Han I, Biau D, Niu X, Bernthal NM, Ferguson PC, Wunder JS. The role of chemotherapy and radiotherapy in localized extraskeletal osteosarcoma. Eur J Cancer, 125:130-141, 2020
11. Nagano A, Matsumoto S, Kawai A, Okuma T, Hiraga H, Matsumoto Y, Nishida Y, Yonemoto T, Hosaka M, Takahashi M, Yoshikawa H, Kunisada T, Asanuma K, Naka N, Emori M, Kubo T, Kawashima H, Kawamoto T, Yokoyama R, Tsukushi S, Sato K, Okamoto T, Hiraoka K, Morioka H, Tanaka K, Takagi T, Iwamoto Y, Ozaki T. Osteosarcoma in patients over 50 years of age: Multi-institutional retrospective analysis of 104 patients. J Orthop Sci, 25:319-323, 2020
12. Kobayashi E, Naito Y, Asano N, Maejima A, Endo M, Takahashi S, Megumi Y, Kawai A. Interim results of a real-world observational study of eribulin in soft tissue sarcoma including rare subtypes. Jpn J Clin Oncol, 49:938-946, 2019
13. Hirata M, Asano N, Katayama K, Yoshida A, Tsuda Y, Sekimizu M, Mitani S, Kobayashi E, Komiyama M, Fujimoto H, Goto T, Iwamoto Y, Naka N, Iwata S, Nishida Y, Hiruma T, Hiraga H, Kawano H, Motoi T, Oda Y, Matsubara D, Fujita M, Shibata T, Nakagawa H, Nakayama R, Kondo T, Imoto S, Miyano S, Kawai A, Yamaguchi R, Ichikawa H, Matsuda K. Integrated exome and RNA sequencing of dedifferentiated liposarcoma. Nat Commun, 10:5683, 2019
14. Toki S, Kobayashi E, Yoshida A, Ogura K, Wakai S, Yoshimoto S, Yonemori K, Kawai A. A clinical comparison between dedifferentiated low-grade osteosarcoma and conventional osteosarcoma. Bone Joint J, 101-B:745-752, 2019
15. Yoshida A, Wakai S, Ryo E, Miyata K, Miyazawa M, Yoshida KI, Motoi T, Ogawa C, Iwata S, Kobayashi E, Watanabe SI, Kawai A, Mori T. Expanding the Phenotypic Spectrum of Mesenchymal Tumors Harboring the EWSR1-CREM Fusion. Am J Surg Pathol, 43:1622-1630, 2019
16. Makise N, Sekimizu M, Kobayashi E, Yoshida H, Fukayama M, Kato T, Kawai A, Ichikawa H, Yoshida A. Low-grade endometrial stromal sarcoma with a novel MEAF6-SUZ12 fusion. Virchows Arch, 475:527-531, 2019
17. Yamazaki F, Nakatani F, Asano N, Wakai S, Sekimizu M, Mitani S, Kubo T, Kawai A, Ichikawa H, Yoshida A. Novel NTRK3 Fusions in Fibrosarcomas of Adults. Am J Surg Pathol, 43:523-530, 2019
18. Yoshida A, Arai Y, Tanzawa Y, Wakai S, Hama N, Kawai A, Shibata T. KMT2A (MLL) fusions in aggressive sarcomas in young adults. Histopathology, 75:508-516, 2019
19. Yoshida KI, Nakano Y, Honda-Kitahara M, Wakai S, Motoi T, Ogura K, Sano N, Shibata T, Okuma T, Iwata S, Kawai A, Ichimura K, Yoshida A. Absence of H3F3A mutation in a subset of malignant giant cell tumor of bone. Mod Pathol, 32:1751-1761, 2019
20. Tsuda Y, Hirata M, Katayama K, Motoi T, Matsubara D, Oda Y, Fujita M, Kobayashi H, Kawano H, Nishida Y, Sakai T, Okuma T, Goto T, Ogura K, Kawai A, Ae K, Anazawa U, Suehara Y, Iwata S, Miyano S, Imoto S, Shibata T, Nakagawa H, Yamaguchi R, Tanaka S, Matsuda K. Massively parallel sequencing of tenosynovial giant cell tumors reveals novel CSF1 fusion transcripts and novel somatic CBL mutations. Int J Cancer, 145:3276-3284, 2019
21. Kinoshita H, Orita S, Yonemoto T, Ishii T, Iwata S, Kamoda H, Tsukanishi T, Inage K, Abe K, Inoue M, Norimoto M, Umimura T, Fujimoto K, Shiga Y, Kanamoto H, Furuya T, Takahashi K, Ohtori S. Successful total en bloc spondylectomy of the L3 vertebra with a paravertebral giant cell tumor following preoperative treatment with denosumab: a case report. J Med Case Rep, 13:116, 2019
22. Ogura K, Uehara K, Akiyama T, Shinoda Y, Iwata S, Tsukushi S, Kobayashi E, Hirose T, Yonemoto T, Endo M, Tanzawa Y, Nakatani F, Kawano H, Tanaka S, Kawai A. Development of a patient-oriented disease specific outcome measure of health-related quality of life (HRQOL) for musculoskeletal oncology patients. J Orthop Sci, 24:539-547, 2019
23. Nakagawa M, Nakatani F, Matsunaga H, Seki T, Endo M, Ogawara Y, Machida Y, Katsumoto T, Yamagata K, Hattori A, Fujita S, Aikawa Y, Ishikawa T, Soga T, Kawai A, Chuman H, Yokoyama N, Fukushima S, Yahiro K, Kimura A, Shimada E, Hirose T, Fujiwara T, Setsu N, Matsumoto Y, Iwamoto Y, Nakashima Y, Kitabayashi I. Selective inhibition of mutant IDH1 by DS-1001b ameliorates aberrant histone modifications and impairs tumor activity in chondrosarcoma. Oncogene, 38:6835-6849, 2019
24. Asano N, Takeshima H, Yamashita S, Takamatsu H, Hattori N, Kubo T, Yoshida A, Kobayashi E, Nakayama R, Matsumoto M, Nakamura M, Ichikawa H, Kawai A, Kondo T, Ushijima T. Epigenetic reprogramming underlies efficacy of DNA demethylation therapy in osteosarcomas. Sci Rep, 9:20360, 2019
25. Tanaka K, Mizusawa J, Naka N, Kawai A, Katagiri H, Hiruma T, Matsumoto Y, Tsuchiya H, Nakayama R, Hatano H, Emori M, Watanuki M, Yoshida Y, Okamoto T, Abe S, Asanuma K, Yokoyama R, Hiraga H, Yonemoto T, Morii T, Ae K, Nagano A, Yoshikawa H, Fukuda H, Ozaki T, Iwamoto Y. Ten-year follow-up results of perioperative chemotherapy with doxorubicin and ifosfamide for high-grade soft-tissue sarcoma of the extremities: Japan Clinical Oncology Group study JCOG0304. BMC Cancer, 19:890, 2019
26. Sanada Y, Harada M, Kunitomi C, Kanatani M, Izumi G, Hirata T, Fujii T, Suzuki N, Morishige KI, Aoki D, Irahara M, Tsugawa K, Tanimoto M, Nishiyama H, Hosoi H, Sugiyama K, Kawai A, Osuga Y. A Japanese nationwide survey on the cryopreservation of embryos, oocytes and ovarian tissue for cancer patients. J Obstet Gynaecol Res, 45:2021-2028, 2019
27. Nishida Y, Kawai A, Toguchida J, Ogose A, Ae K, Kunisada T, Matsumoto Y, Matsunobu T, Takahashi K, Nishida K, Ozaki T. Clinical features and treatment outcome of desmoid-type fibromatosis: based on a bone and soft tissue tumor registry in Japan. Int J Clin Oncol, 24:1498-1505, 2019
28. Asano N, Matsuzaki J, Ichikawa M, Kawauchi J, Takizawa S, Aoki Y, Sakamoto H, Yoshida A, Kobayashi E, Tanzawa Y, Nakayama R, Morioka H, Matsumoto M, Nakamura M, Kondo T, Kato K, Tsuchiya N, Kawai A, Ochiya T. A serum microRNA classifier for the diagnosis of sarcomas of various histological subtypes. Nat Commun, 10:1299, 2019
29. Urakawa H, Mizusawa J, Tanaka K, Eba J, Hiraga H, Kawai A, Nishida Y, Hosaka M, Iwamoto Y, Fukuda H, Ozaki T. A randomized phase III trial of denosumab before curettage for giant cell tumor of bone: Japan Clinical Oncology Group Study JCOG1610. Jpn J Clin Oncol, 49:379-382, 2019
30. Takeuchi A, Nomura A, Yamamoto N, Hayashi K, Igarashi K, Tandai S, Kawai A, Matsumine A, Miwa S, Nishida Y, Nakamura T, Terauchi R, Hoshi M, Kunisada T, Endo M, Yoshimura K, Murayama T, Tsuchiya H. Randomized placebo-controlled double-blind phase II study of zaltoprofen for patients with diffuse-type and unresectable localized tenosynovial giant cell tumors: a study protocol. BMC Musculoskelet Disord, 20:68, 2019
31. Tashiro K, Arikawa M, Kagaya Y, Kobayashi E, Kawai A, Miyamoto S. Flap reconstruction after groin and medial thigh sarcoma resection reduces the risk of lower-extremity lymphedema. J Plast Reconstr Aesthet Surg, 72:685-710, 2019
32. Koike H, Nishida Y, Kohno K, Shimoyama Y, Motoi T, Hamada S, Kawai A, Ogose A, Ozaki T, Kunisada T, Matsumoto Y, Matsunobu T, Ae K, Gokita T, Sakai T, Shimizu K, Ishiguro N. Is immunohistochemical staining for β-catenin the definitive pathological diagnostic tool for desmoid-type fibromatosis? A multi-institutional study. Hum Pathol, 84:155-163, 2019
33. Makise N, Sekimizu M, Konishi E, Motoi T, Kubo T, Ikoma H, Watanabe SI, Okuma T, Hiraoka N, Fukayama M, Kawai A, Ichikawa H, Yoshida A. H3K27me3 deficiency defines a subset of dedifferentiated chondrosarcomas with characteristic clinicopathological features. Mod Pathol, 32:435-445, 2019
34. Tashiro K, Arikawa M, Fukunaga Y, Nakatani F, Kobayashi E, Kawai A, Miyamoto S. Free latissimus dorsi musculocutaneous flap for external hemipelvectomy reconstruction. Microsurgery, 39:138-143, 2019
