Annual Report 2022
Division of Radiation Oncology and Particle Therapy
Tetsuo Akimoto, Sadatomo Zenda, Atsushi Motegi, Hidenobu Tachibana, Kenji Hotta, Hiromi Baba, Kana Motegi, Masaki Nakamura, Hidehiro Hojo, Ryo Takahashi, Shunichiro Kageyama, Hidenari Hirata
Introduction
The aim of research in the Division of Radiation Oncology and Particle Therapy at the National Cancer Center Hospital East (NCCHE) is to study and develop innovative treatment techniques and pilot a clinical trial for proton beam therapy (PBT). Medical physicists mainly perform the development and verification of the systems for beam irradiation, dose calculation, dose measurement, and imaging of PBT. Radiation oncologists mainly perform studies on the clinical trials, efficacy, and side-effects of PBT.
The Team and What We Do
At present, the staff of the Division of Radiation Oncology and Particle Therapy consists of seven consultant physicians (radiation oncologists), six radiation technologists, four medical physicists, one nurse, and one clerk. We have more than 300 new patients for PBT every year. Quality assurance of PBT is performed by medical physicists and radiation technologists, and a conference to verify the treatment planning is held every morning in addition to a weekly work conference on research activities. PBT is routinely based on three-dimensional radiation therapy planning and PBT using RT-dedicated multi-detector-row helical computed tomography (CT) scanning to confirm the precise radiation dose to be administered to the targeted tumors. Respiratory gating has been employed especially in the radiotherapeutic management of patients with lung, esophagus, and liver cancers.
Our division is responsible for PBT and is composed of seven operating staff members and one technician for fabricating the compensator and aperture; they are sent from manufacturing companies and work in collaboration with other staff members of our division. PBT consists of two treatment rooms, both of which are routinely used for rotational gantry treatment. Our division ensures quality assurance and regular maintenance of the PBT machines for precise dose delivery and safe treatment.
Research Activities
1. Phase I/II study of dose escalated PBT combined with chemotherapy for esophageal cancer.
2. Non-randomized prospective comparative study between surgical resection and proton beam therapy for resectable hepatocellular carcinoma.
3. Establishment of the feasibility and effectiveness of line scanning for localized prostate cancer.
4. Proton dose distribution measurements using a MOSFET detector with a simple dose-weighted correction method for LET effects.
5. Radiobiological evaluation of the cellular response to PBT.
6. Radiobiological evaluation of the combined effect of chemotherapeutic agents on enhancement of PBT.
7. Standardization of methods of PBT and quality assurance of PBT among Japanese proton beam facilities.
8. Establishment of infrastructure for multi-institutional study of PBT for various cancers. Technical development of intensity modulated proton beam therapy (IMPT).
9. In silico comparison of dose distribution between IMRT and IMPT for locally advanced head and neck squamous cell carcinoma.
10. Standardization and clinical implementation of the model-based approach for the evaluation of new treatment technologies.
Clinical Trials
The following in-house and multi-institutional clinical trials are ongoing:
1) Phase I/II study of dose escalated PBT combined with chemotherapy for esophageal cancer.
2) JCOG1315C: Non-randomized prospective comparative study between surgical resection and proton beam therapy for resectable hepatocellular carcinoma.
3) Pilot study of IMPT for locally advanced head and neck squamous cell carcinoma.
4) iProron study: Multi-institutional study of IMPT combined with concurrent chemotherapy for locally advanced head and neck squamous cell carcinoma.
Education
We established an education and training system for residents and junior radiation oncologists through clinical conferences and lectures on radiation oncology, physics, and radiation biology. In addition, a training course on quality assurance of radiation therapy including PBT has been regularly held for medical physicists and radiological technologists.
Future Prospects
We are now aiming to establish a system that can provide high-quality and safe PBT. In addition, we would like to promote the research and development of innovative technologies related to PBT, radiation biology, and medical physics.
Table 1. Number of patients treated with PBT during 2018-2022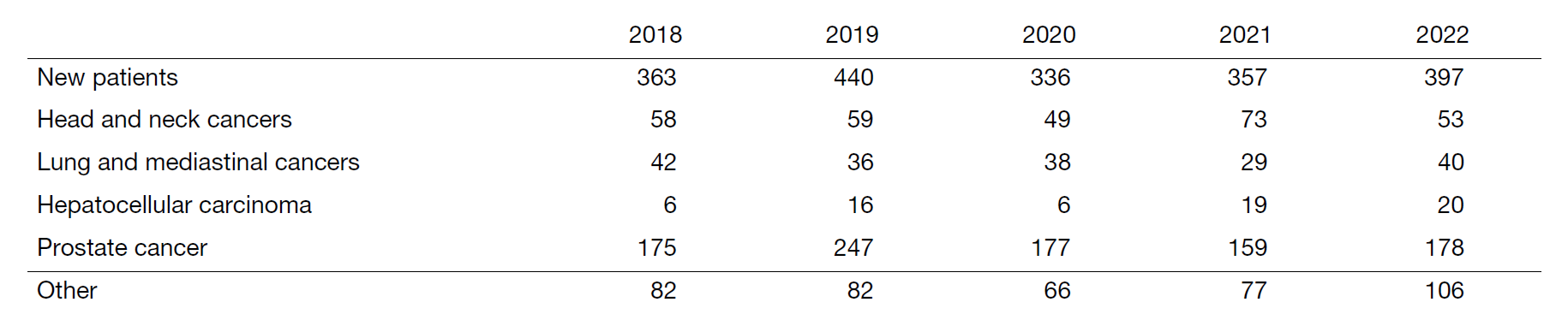

List of papers published in 2022
Journal
1. Zenda S, Arai Y, Sugawara S, Inaba Y, Hashimoto K, Yamamoto K, Saigusa Y, Kawaguchi T, Shimada S, Yokoyama M, Miyaji T, Okano T, Nakamura N, Kobayashi E, Takagi T, Matsumoto Y, Uchitomi Y, Sone M. Protocol for a confirmatory trial of the effectiveness and safety of palliative arterial embolization for painful bone metastases. BMC cancer, 23:109, 2023
2. Sekiguchi K, Sumi M, Saito A, Zenda S, Arahira S, Iino K, Okumura M, Kawai F, Nozawa K. The effectiveness of moisturizer on acute radiation-induced dermatitis in breast cancer patients: a systematic review and meta-analysis. Breast cancer (Tokyo, Japan), 30:2-12, 2023
3. Sekiguchi K, Sumi M, Saito A, Zenda S, Arahira S, Iino K, Okumura M, Kawai F, Nozawa K. Correction to: The effectiveness of moisturizer on acute radiation-induced dermatitis in breast cancer patients: a systematic review and meta-analysis. Breast cancer (Tokyo, Japan), 30:13, 2023
4. Kawamura H, Nakamura K, Yoshioka Y, Itasaka S, Tomita N, Onishi M, Iwata H, Aizawa T, Kikuchi K, Nagata K, Nakamura K, Nishioka K, Ishiyama H, Ueno S, Kokubo M, Yamazaki H, Watanabe K, Toyoda T, Akimoto T. Radiotherapy for ductal carcinoma of the prostate: an analysis based on the Japanese radiation oncology study group survey. Japanese journal of clinical oncology, 53:146-152, 2023
5. Endo M, Kawahara S, Sato T, Tokunaga M, Hara T, Mawatari T, Kawano T, Zenda S, Miyaji T, Shimokawa M, Sakamoto S, Takano T, Miyake M, Aono H, Nakashima Y. Protocol for the RETHINK study: a randomised, double-blind, parallel-group, non-inferiority clinical trial comparing acetaminophen and NSAIDs for treatment of chronic pain in elderly patients with osteoarthritis of the hip and knee. BMJ open, 13:e068220, 2023
6. Nozaki M, Kagami Y, Takahashi M, Machida R, Sekino Y, Shibata T, Ito Y, Nishimura Y, Teshima T, Ushijima H, Nagata Y, Matsumoto Y, Akimoto T, Takahashi K, Murayama S, Uno T, Tsujino K, Hamamoto Y, Nakagawa K, Kodaira T, Hiraoka M. Evaluation of breast cosmetic changes with a computer-software; the breast cancer conservative treatment cosmetic results (BCCT. core) in hypofractionated whole breast irradiation after breast-conserving surgery-supplementary analysis of multicenter single-arm confirmatory trial: JCOG0906. Breast cancer (Tokyo, Japan), 29:1042-1049, 2022
7. Okumura M, Du J, Kageyama SI, Yamashita R, Hakozaki Y, Motegi A, Hojo H, Nakamura M, Hirano Y, Okuma Y, Okuma HS, Tsuchihara K, Akimoto T. Comprehensive screening for drugs that modify radiation-induced immune responses. British journal of cancer, 126:1815-1823, 2022
8. Ito R, Nakamura Y, Sunakawa H, Fujiwara H, Hojo H, Nakamura N, Fujita T, Yano T, Daiko H, Akimoto T, Yoshino T, Kojima T. Tumor response and survival outcomes of salvage concurrent chemoradiotherapy with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy for postoperative locoregional recurrence of esophageal squamous cell carcinoma. Esophagus, 19:645-652, 2022
9. Yokota T, Ueno T, Soga Y, Ishiki H, Uezono Y, Mori T, Zenda S, Uchitomi Y. J-SUPPORT research policy for oral mucositis associated with cancer treatment. Cancer medicine, 11:4816-4829, 2022
10. Yaguchi-Saito A, Kaji Y, Matsuoka A, Okuyama A, Fujimori M, Saito J, Odawara M, Otsuki A, Uchitomi Y, Zenda S, Shimazu T. Factors affecting the implementation of guideline-based prophylactic antiemetic therapy for chemotherapy-induced nausea and vomiting in Japan: a protocol for a hospital-based qualitative study. BMJ open, 12:e055473, 2022
11. Nakajo K, Inaba A, Aoyama N, Takashima K, Kadota T, Yoda Y, Morishita Y, Okano W, Tomioka T, Shinozaki T, Matsuura K, Hayashi R, Akimoto T, Yano T. The characteristics of missed pharyngeal and laryngeal cancers at gastrointestinal endoscopy. Japanese journal of clinical oncology, 52:575-582, 2022
12. Takeshita N, Enokida T, Okano S, Fujisawa T, Wada A, Sato M, Tanaka H, Tanaka N, Motegi A, Zenda S, Akimoto T, Tahara M. Induction chemotherapy with paclitaxel, carboplatin and cetuximab for locoregionally advanced nasopharyngeal carcinoma: A single-center, retrospective study. Frontiers in oncology, 12:951387, 2022
13. Sakai SA, Aoshima M, Sawada K, Horasawa S, Yoshikawa A, Fujisawa T, Kadowaki S, Denda T, Matsuhashi N, Yasui H, Goto M, Yamazaki K, Komatsu Y, Nakanishi R, Nakamura Y, Bando H, Hamaya Y, Kageyama SI, Yoshino T, Tsuchihara K, Yamashita R. Fecal microbiota in patients with a stoma decreases anaerobic bacteria and alters taxonomic and functional diversities. Frontiers in cellular and infection microbiology, 12:925444, 2022
14. Mizuno M, Chiba I, Mukohara T, Kondo M, Maruo K, Ohigashi T, Naruo M, Asano Y, Onishi T, Tanabe H, Muta R, Mishima S, Okano S, Yuda M, Hosono A, Ueda Y, Bando H, Itagaki H, Ferrans CE, Akimoto T. Effectiveness of an online support program to help female cancer patients manage their health and illness: Protocol for a randomized controlled trial. Contemporary clinical trials communications, 30:101035, 2022
15. Du J, Kageyama SI, Yamashita R, Hirata H, Hakozaki Y, Okumura M, Motegi A, Hojo H, Nakamura M, Hirano Y, Sunakawa H, Minamide T, Kotani D, Tanaka K, Yano T, Kojima T, Ohashi A, Tsuchihara K, Akimoto T. Impacts of the STING-IFNAR1-STAT1-IRF1 pathway on the cellular immune reaction induced by fractionated irradiation. Cancer science, 113:1352-1361, 2022
16. Nakajo K, Yoda Y, Yamashita H, Takashima K, Murano T, Kadota T, Shinmura K, Ikematsu H, Akimoto T, Yano T. Salvage endoscopic resection for cT1N0M0 local recurrence after chemoradiotherapy for esophageal squamous cell carcinoma: endoscopic submucosal dissection versus endoscopic mucosal resection. Japanese journal of clinical oncology, 52:982-991, 2022
17. Takahashi S, Ohno I, Ikeda M, Konishi M, Kobayashi T, Akimoto T, Kojima M, Morinaga S, Toyama H, Shimizu Y, Miyamoto A, Tomikawa M, Takakura N, Takayama W, Hirano S, Otsubo T, Nagino M, Kimura W, Sugimachi K, Uesaka K. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Annals of surgery, 276:e510-e517, 2022
18. Akiyama N, Okamura T, Yoshida M, Kimura SI, Yano S, Yoshida I, Kusaba H, Takahashi K, Fujita H, Fukushima K, Iwasaki H, Tamura K, Saeki T, Takamatsu Y, Zenda S. Difference of compliance rates for the recommendations in Japanese Guideline on Febrile Neutropenia according to respondents’ attributes: the second report on a questionnaire survey among hematology-oncology physicians and surgeons. Supportive care in cancer, 30:4327-4336, 2022
19. Okumura M, Hojo H, Akimoto T. Response to palliative radiotherapy for cancer patients with interstitial lung disease: A physician’s perspective. Radiotherapy and oncology, 169:157-158, 2022
20. Tamaki Y, Aibe N, Komiyama T, Nagasaka S, Imagumbai T, Itazawa T, Onishi H, Akimoto T, Nagata Y, Nakayama Y. Optimal Clinical Target Volume of Radiotherapy Based on Microscopic Extension around the Primary Gross Tumor in Non-Small-Cell Lung Cancer: A Systematic Review. Cancers, 14:2318, 2022
21. Yamaguchi T, Sugiyama Y, Tanaka T, Kimura T, Yumura Y, Nakano M, Sugiyama T, Miura N, Goya M, Yamamoto A, Takahashi S, Miura Y, Tsuzuki T, Masumori N, Nishiyama H, Yao M, Koie T, Miyake H, Saika T, Saito S, Akimoto T, Tamada T, Ando Y, Takahashi S, Suzuki T, Hinotsu S, Kamba T. Summary of the Clinical Practice Guidelines for Penile Cancer 2021 by the Japanese Urological Association. International journal of urology, 29:780-792, 2022
22. Tachibana H, Takahashi R, Kogure T, Nishiyama S, Kurosawa T. Practical dosimetry procedure of air kerma for kilovoltage X-ray imaging in radiation oncology using a 0.6-cc cylindrical ionization chamber with a cobalt absorbed dose-to-water calibration coefficient. Radiological physics and technology, 15:264-270, 2022
23. Tachibana H, Watanabe Y, Kurokawa S, Maeyama T, Hiroki T, Ikoma H, Hirashima H, Kojima H, Shiinoki T, Tanimoto Y, Shimizu H, Shishido H, Oka Y, Hirose TA, Kinjo M, Morozumi T, Kurooka M, Suzuki H, Saito T, Fujita K, Shirata R, Inada R, Yada R, Yamashita M, Kondo K, Hanada T, Takenaka T, Usui K, Okamoto H, Asakura H, Notake R, Kojima T, Kumazaki Y, Hatanaka S, Kikumura R, Nakajima M, Nakada R, Suzuki R, Mizuno H, Kawamura S, Nakamura M, Akimoto T. Multi-Institutional Study of End-to-End Dose Delivery Quality Assurance Testing for Image-Guided Brachytherapy Using a Gel Dosimeter. Brachytherapy, 21:956-967, 2022
24. Ota Y, Kodaira T, Fujii H, Shimokawa M, Yokota T, Nakashima T, Monden N, Homma A, Ueda S, Akimoto T. Real-world clinical outcomes in Japanese patients with locally advanced squamous cell carcinoma of the head and neck treated with radiotherapy plus cetuximab: a prospective observational study (JROSG12-2). International journal of clinical oncology, 27:1675-1683, 2022
25. Watanabe Y, Maeyama T, Mizukami S, Tachibana H, Terazaki T, Takei H, Muraishi H, Gomi T, Hayashi SI. Verification of dose distribution in high dose-rate brachytherapy for cervical cancer using a normoxic N-vinylpyrrolidone polymer gel dosimeter. Journal of radiation research, 63:838-848, 2022
26. Matsumura H, Yoshida G, Toyoda A, Masumoto K, Nakamura H, Miura T, Nishikawa K, Bessho K, Akita T, Katsuta S, Akimoto T, Sugama Y, Nobuhara F, Nagashima Y. Investigation of Concrete Radioactivation in Cyclotron Type Proton Therapy Facilities using in situ 24Na Measurement Method. Radiation Safety Management, 21:13-25, 2022
