Annual Report 2022
Department of Thoracic Oncology
Yuichiro Ohe, Noboru Yamamoto, Hidehito Horinouchi, Yasushi Goto, Tatsuya Yoshida, Yusuke Okuma, Yuki Shinno, Ken Masuda, Yuji Matsumoto, Masahiro Torasawa,Yukiko Shimoda, Akiko Tateishi
Introduction
Lung cancer is the leading cause of cancer death in Japan and worldwide. The incidence of lung cancer in Japan is still increasing, especially in the elderly. The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The goals of the department are to provide treatment with the highest quality and to establish new effective treatments against lung cancer and other thoracic malignancies through innovative clinical and translational research. To assist our patients through multidisciplinary care, the staff members of the department work closely with thoracic surgeons, radiation oncologists, pathologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. The department includes nine staff physicians. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
The Team and What We Do
The staff physicians attend outpatient services for thoracic diseases, and the department has approximately 50 beds in the hospital. Inpatient care is carried out by five teams. Each team consists of one staff physician and one or two residents and/or trainee doctors. Case conferences are scheduled every Monday and Tuesday morning. Protocol conference and journal club are scheduled every Monday afternoon and Thursday morning, respectively.
A total of 570 new patients started treatment in 2022, and their backgrounds and initial treatments of these patients are shown in Tables 1 and 2. The initial treatments were chemotherapy in 285 cases, adjuvant chemotherapy after surgery in 67, chemoradiotherapy in 76, curative radiotherapy in 10, and supportive care including palliative radiotherapy in 60. The survival rates of lung cancer patients treated in 2012-2016 in our department is shown in Table 3.
Table 1. Number of patients in 2022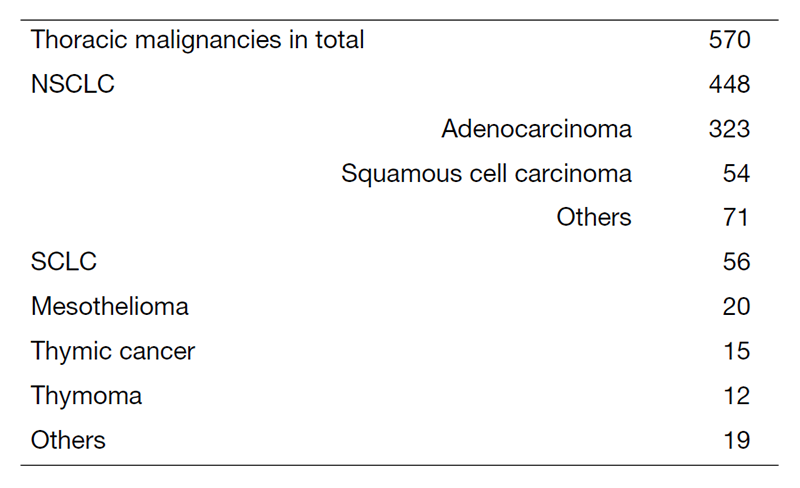

Table 2. Type of procedure in 2022

Table 3. Survival rates of lung cancer patients treated in 2012-2016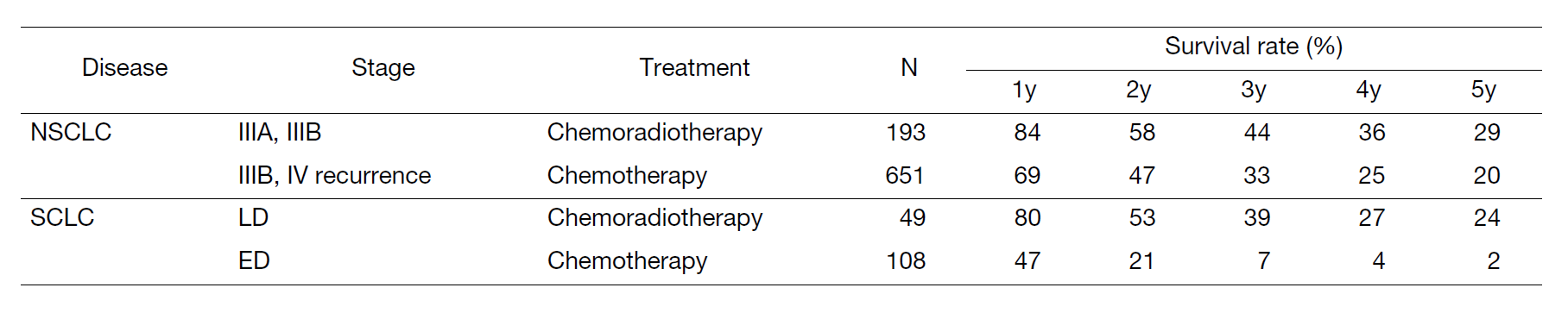

Research Activities
Research activities of the department can be classified into four categories: (1) multi-institutional phase III studies to establish new standard treatments against lung cancer; (2) phase I and II studies to evaluate new anticancer drugs; (3) pharmacokinetic and pharmacodynamic (PK/PD) studies to investigate interpatient variability, optimal administration schedules and drug-drug interactions; and (4) translational research using clinical samples from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies.
Clinical Trials
The department is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments against lung cancer such as the Japan Clinical Oncology Group (JCOG) trials and global trials conducted by pharmaceutical companies. Six JCOG clinical studies, namely JCOG1408 (J-SBRT trial), a phase III study of SBRT for c-stage IA NSCLC, JCOG1701 (SAVE study), a phase III study of immune checkpoint inhibitors to evaluate optimal treatment period, JCOG1807C (DEEP OCEAN), chemoradiotherapy with durvalumab and surgery for superior sulcus NSCLC, JCOG1914, a phase III of chemoradiotherapy for locally advanced NSCLC in the elderly, JCOG2002 (yES-TRT), phase III study of thoracic radiotherapy for ED-SCLC after chemotherapy with immune checkpoint inhibitor (ICI), JCOG2007 (NIPPON), a phase III study of chemotherapy with ICIs for NSCLC, are ongoing. The department is also participating in a nationwide screening project of lung cancer with rare driver mutation (LC-SCRUM). Moreover, the department has conducted many clinical trials using TKIs, ICIs, antibody-drug conjugates (ADC) and bispecific antibodies.
Education
In 2022, 3 chief residents, 11 residents, and 9 trainee doctors joined the department. A monthly research conference is held to discuss the clinical and translational research conducted by young doctors.
Future Prospects
Recent progression of lung cancer treatment is very rapid. The driver gene alteration targeted therapy, such as EGFR-TKIs for EGFR mutation positive lung cancer, ALK inhibitors for ALK fusion gene-positive lung cancer, ROS1 inhibitors for ROS1 fusion gene-positive lung cancer, BRAF plus MEK inhibitor for BRAF V600E-positive lung cancer, NTRK inhibitors for NTRK fusion-positive lung cancer, RET inhibitor for RET fusion-positive lung cancer, MET inhibitors for MET exon 14 skipping mutation-positive lung cancer and KRAS inhibitor for KRAS G12C lung cancer have already been established as the standard treatment. Other rare driver gene alterations, including HER2, NRG and CLIP-LTK fusion will be good targets for the treatment of lung cancer. Immune checkpoint inhibitors, anti-PD-1 Ab and anti-PD-L1 Ab plus chemotherapy have been established as a standard 1st line treatment for NSCLC. A combination of anti-PD-1 Ab and anti-CTLA-4 Ab with or without chemotherapy has also established as a new standard treatment for advanced NSCLC. Anti-PD-L1 Ab, durvalumab for stage III NSCLC after chemoradiotherapy will also be established as a standard treatment. An immune checkpoint inhibitor will also be an incorporated treatment for early stage lung cancer with surgery or SBRT. Moreover, antibody-drug conjugates (ADC) and bispecific antibodies will now be treatment options for lung cancer patients.
List of papers published in 2022
Journal
1. Yazaki S, Shimoi T, Yoshida M, Sumiyoshi-Okuma H, Arakaki M, Saito A, Kita S, Yamamoto K, Kojima Y, Nishikawa T, Tanioka M, Sudo K, Noguchi E, Murata T, Shiino S, Takayama S, Suto A, Ohe Y, Fujiwara Y, Yonemori K. Integrative prognostic analysis of tumor-infiltrating lymphocytes, CD8, CD20, programmed cell death-ligand 1, and tertiary lymphoid structures in patients with early-stage triple-negative breast cancer who did not receive adjuvant chemotherapy. Breast cancer research and treatment, 197:287-297, 2023
2. Yazaki S, Salgado R, Shimoi T, Yoshida M, Shiino S, Kaneda T, Kojima Y, Sumiyoshi-Okuma H, Nishikawa T, Sudo K, Noguchi E, Murata T, Takayama S, Suto A, Ohe Y, Yonemori K. Impact of adjuvant chemotherapy and radiotherapy on tumour-infiltrating lymphocytes and PD-L1 expression in metastatic breast cancer. British journal of cancer, 128:568-575, 2023
3. Matsuzaki J, Kato K, Oono K, Tsuchiya N, Sudo K, Shimomura A, Tamura K, Shiino S, Kinoshita T, Daiko H, Wada T, Katai H, Ochiai H, Kanemitsu Y, Takamaru H, Abe S, Saito Y, Boku N, Kondo S, Ueno H, Okusaka T, Shimada K, Ohe Y, Asakura K, Yoshida Y, Watanabe SI, Asano N, Kawai A, Ohno M, Narita Y, Ishikawa M, Kato T, Fujimoto H, Niida S, Sakamoto H, Takizawa S, Akiba T, Okanohara D, Shiraishi K, Kohno T, Takeshita F, Nakagama H, Ota N, Ochiya T. Prediction of tissue-of-origin of early stage cancers using serum miRNomes. JNCI cancer spectrum, 7:pkac080, 2023
4. Higashiyama M, Motoi N, Yotsukura M, Yoshida Y, Nakagawa K, Yagishita S, Shirasawa M, Yoshida T, Shiraishi K, Kohno T, Ohe Y, Watanabe SI. Clinicopathological characteristics and molecular analysis of lung cancer associated with ciliated muconodular papillary tumor/bronchiolar adenoma. Pathology international, 73:188-197, 2023
5. Udagawa H, Takahashi S, Hirao M, Tahara M, Iwasa S, Sato Y, Hamakawa T, Shitara K, Horinouchi H, Chin K, Masuda N, Suzuki T, Okumura S, Takase T, Nagai R, Yonemori K. Liposomal eribulin for advanced adenoid cystic carcinoma, gastric cancer, esophageal cancer, and small cell lung cancer. Cancer medicine, 12:1269-1278, 2023
6. Iwasa S, Koyama T, Nishino M, Kondo S, Sudo K, Yonemori K, Yoshida T, Tamura K, Shimizu T, Fujiwara Y, Kitano S, Shimomura A, Sato J, Yokoyama F, Iida H, Kondo M, Yamamoto N. First-in-human study of ONO-4578, an antagonist of prostaglandin E(2) receptor 4, alone and with nivolumab in solid tumors. Cancer science, 114:211-220, 2023
7. Yoshimura A, Yamada T, Serizawa M, Uehara H, Tanimura K, Okuma Y, Fukuda A, Watanabe S, Nishioka N, Takeda T, Chihara Y, Takemoto S, Harada T, Hiranuma O, Shirai Y, Shukuya T, Nishiyama A, Goto Y, Shiotsu S, Kunimasa K, Morimoto K, Katayama Y, Suda K, Mitsudomi T, Yano S, Kenmotsu H, Takahashi T, Takayama K. High levels of AXL expression in untreated EGFR-mutated non-small cell lung cancer negatively impacts the use of osimertinib. Cancer science, 114:606-618, 2023
8. Noda-Narita S, Naito T, Udagawa H, Goto K, Miyawaki T, Mamesaya N, Nakashima K, Kenmotsu H, Shimokawaji T, Kato T, Hakozaki T, Okuma Y, Nakamura M, Nakayama Y, Watanabe H, Kusumoto M, Ohe Y, Horinouchi H. Nivolumab-induced radiation recall pneumonitis in non-small-cell lung cancer patients with thoracic radiation therapy. Cancer science, 114:630-639, 2023
9. Goto Y, Yoh K, Kato T, Hosomi Y, Usui K, Fukui T, Hirano K, Tanaka H, Taguri M, Kunitoh H. Observational study to predict the efficacy and optimal duration of nivolumab treatment in patients with previously treated advanced or recurrent non-small cell lung cancer. Japanese journal of clinical oncology, 53:153-160, 2023
10. Nishimura R, Yoshida T, Torasawa M, Kashihara T, Ohe Y. Co-occurring KEAP1 and TP53 mutations in lung squamous cell carcinoma induced primary resistance to thoracic radiotherapy: A case report. Thoracic cancer, 14:206-209, 2023
11. Tanaka T, Yoshida T, Masuda K, Takeyasu Y, Shinno Y, Matsumoto Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Ohe Y. Prognostic role of modified Glasgow Prognostic score in elderly non-small cell lung cancer patients treated with anti-PD-1 antibodies. Respiratory investigation, 61:74-81, 2023
12. Takamizawa S, Katsuya Y, Chen YN, Mizuno T, Koyama T, Sudo K, Yoshida T, Kondo S, Iwasa S, Yonemori K, Shimizu T, Yamamoto N, Suzuki S. Ocular toxicity of investigational anti-cancer drugs in early phase clinical trials. Investigational new drugs, 41:173-181, 2023
13. Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F, Goto Y, Kim DW, Wu YL, Jassem J, López FL, Soo RA, Shaw AT, Polli A, Messina R, Iadeluca L, Toffalorio F, Felip E. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. The Lancet. Respiratory medicine, 11:354-366, 2023
14. Konno-Yamamoto A, Matsumoto Y, Imabayashi T, Tanaka M, Uchimura K, Nakagomi T, Yanase K, So C, Ohe Y, Tsuchida T. Feasibility of Modified Endobronchial Ultrasound-Guided Intranodal Forceps Biopsy: A Retrospective Analysis. Respiration; international review of thoracic diseases, 102:143-153, 2023
15. Goto K, Shiraishi Y, Murakami H, Horinouchi H, Toyozawa R, Takeda M, Uno M, Crawford N, McGill J, Jimbo T, Ishigami M, Takayama G, Nakayama S, Ohwada S, Nishio M. Phase 1 study of DS-1205c combined with gefitinib for EGFR mutation-positive non-small cell lung cancer. Cancer medicine, 12:7090-7104, 2023
16. Kashihara T, Nakayama Y, Okuma K, Takahashi A, Kaneda T, Katagiri M, Nakayama H, Kubo Y, Ito K, Nakamura S, Takahashi K, Inaba K, Murakami N, Saito T, Okamoto H, Itami J, Kusumoto M, Ohe Y, Igaki H. Impact of interstitial lung abnormality on survival after adjuvant durvalumab with chemoradiotherapy for locally advanced non-small cell lung cancer. Radiotherapy and oncology, 180:109454, 2023
17. Higashiyama RI, Yoshida T, Yagishita S, Hamada A. In Response: Letter Received From Dr. Charles Ricordel Titled "Safety of Extended-Interval Dosing Strategy of Immune Checkpoint Inhibitors for Advanced NSCLC". Journal of thoracic oncology, 18:e16-e17, 2023
18. Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N, Blackhall F, Dowlati A, Reguart N, Yoshida T, He K, Gadgeel SM, Felip E, Zhang Y, Pati A, Minocha M, Mukherjee S, Goldrick A, Nagorsen D, Hashemi Sadraei N, Owonikoko TK. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. Journal of clinical oncology, 41:2893-2903, 2023
19. Fukuda A, Yoshida T, Yagishita S, Shiotsuka M, Kobayashi O, Iwata S, Umeguchi H, Yanagida M, Irino Y, Masuda K, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Hamada A, Yamamoto N, Ohe Y. Real-world Data on the Incidence of Coronavirus Disease (COVID-19) in Patients With Advanced Thoracic Cancer During the Early Phase of the Pandemic in Japan. Anticancer research, 43:919-926, 2023
20. Fujiwara Y, Makihara R, Hase T, Hashimoto N, Naito T, Tsubata Y, Okuno T, Takahashi T, Kobayashi H, Shinno Y, Zenke Y, Ikeda T, Hosomi Y, Watanabe K, Kitazono S, Sakiyama N, Makino Y, Yamamoto N. Pharmacokinetic and dose-finding study of osimertinib in patients with impaired renal function and low body weight. Cancer science, 114:2087-2097, 2023
21. Takeshita K, Hijioka S, Nagashio Y, Maruki Y, Kawasaki Y, Maehara K, Murashima Y, Okada M, Ikeda G, Yamada N, Takasaki T, Agarie D, Hara H, Hagiwara Y, Okamoto K, Yamashige D, Ohba A, Kondo S, Morizane C, Ueno H, Saito Y, Ohe Y, Okusaka T. Diagnostic Ability of Endoscopic Ultrasound-Guided Tissue Acquisition Using 19-Gauge Fine-Needle Biopsy Needle for Abdominal Lesions. Diagnostics (Basel, Switzerland), 13:450, 2023
22. Garassino MC, Gadgeel S, Novello S, Halmos B, Felip E, Speranza G, Hui R, Garon EB, Horinouchi H, Sugawara S, Rodriguez-Abreu D, Reck M, Cristescu R, Aurora-Garg D, Loboda A, Lunceford J, Kobie J, Ayers M, Piperdi B, Pietanza MC, Paz-Ares L. Associations of Tissue Tumor Mutational Burden and Mutational Status With Clinical Outcomes With Pembrolizumab Plus Chemotherapy Versus Chemotherapy For Metastatic NSCLC. JTO clinical and research reports, 4:100431, 2023
23. Chmielecki J, Gray JE, Cheng Y, Ohe Y, Imamura F, Cho BC, Lin MC, Majem M, Shah R, Rukazenkov Y, Todd A, Markovets A, Barrett JC, Hartmaier RJ, Ramalingam SS. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nature communications, 14:1070, 2023
24. Goto Y, Shukuya T, Murata A, Kikkawa H, Emir B, Wiltshire R, Miura S. Real-world therapeutic effectiveness of lorlatinib after alectinib in Japanese patients with ALK-positive non-small-cell lung cancer. Cancer science, 114:2560-2568, 2023
25. Nagasaka M, Ohe Y, Zhou C, Choi CM, Yang N, Liu G, Felip E, Pérol M, Besse B, Nieva J, Raez L, Pennell NA, Dimou A, Marinis F, Ciardiello F, Seto T, Hu Z, Pan M, Wang W, Li S, Ou SI. TRUST-II: a global phase II study of taletrectinib in ROS1-positive non-small-cell lung cancer and other solid tumors. Future oncology (London, England), 19:123-135, 2023
26. Furuse H, Matsumoto Y, Nakai T, Tanaka M, Nishimatsu K, Uchimura K, Imabayashi T, Tsuchida T, Ohe Y. Diagnostic efficacy of cryobiopsy for peripheral pulmonary lesions: A propensity score analysis. Lung cancer (Amsterdam, Netherlands), 178:220-228, 2023
27. Akagi K, Yagishita S, Ohuchi M, Hayashi Y, Takeyasu Y, Masuda K, Shinno Y, Okuma Y, Yoshida T, Goto Y, Horinouchi H, Yamamoto N, Mukae H, Ohe Y, Hamada A. Impact of ramucirumab pharmacokinetics in combination with docetaxel on the efficacy and survival in patients with advanced non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands), 178:247-253, 2023
28. Jo H, Yoshida T, Yagishita S, Ohuchi M, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Takahashi K, Motoi N, Hamada A, Ohe Y. Clinical Characteristics and Pharmacokinetics Change of Long-Term Responders to Antiprogrammed Cell Death Protein 1 Inhibitor Among Patients With Advanced NSCLC. JTO clinical and research reports, 4:100474, 2023
29. Hayashi H, Teraoka S, Goto Y, Kumagai T, Nishio M, Sugawara S, Oizumi S, Matsumura M, Okura M, Peltz G, Kato T. First-Line Lorlatinib Versus Crizotinib in ALK-Positive NSCLC: Japanese Subgroup Analysis of CROWN. JTO clinical and research reports, 4:100471, 2023
30. Higashiyama RI, Horinouchi H, Kuchiba A, Matsumoto Y, Murakami S, Goto Y, Kanda S, Fujiwara Y, Yamamoto N, Ohe Y. Non-specific symptoms as a prodrome of immune-related adverse events in patients with non-small cell lung cancer receiving nivolumab: a consecutive analysis of 200 patients. Journal of cancer research and clinical oncology, 2022
31. Takemura C, Kashima J, Hashimoto T, Ichikawa H, Honma Y, Goto Y, Watanabe SI, Yatabe Y. A mimic of lung adenocarcinoma: a case report of histological conversion of metastatic thyroid papillary carcinoma. Histopathology, 80:1004-1007, 2022
32. Kobayashi AK, Nakagawa K, Nakayama Y, Ohe Y, Yotsukura M, Uchida S, Asakura K, Yoshida Y, Watanabe SI. Salvage Surgery Compared to Surgery After Induction Chemoradiation Therapy for Advanced Lung Cancer. The Annals of thoracic surgery, 114:2087-2092, 2022
33. Aokage K, Tsuboi M, Zenke Y, Horinouchi H, Nakamura N, Ishikura S, Nishikawa H, Kumagai S, Koyama S, Kanato K, Kataoka T, Wakabayashi M, Fukutani M, Fukuda H, Ohe Y, Watanabe SI. Study protocol for JCOG1807C (DEEP OCEAN): a interventional prospective trial to evaluate the efficacy and safety of durvalumab before and after operation or durvalumab as maintenance therapy after chemoradiotherapy against superior sulcus non-small cell lung cancer. Japanese journal of clinical oncology, 52:383-387, 2022
34. Kashima J, Hashimoto T, Yoshida A, Goto Y, Ushiku T, Ohe Y, Watanabe SI, Yatabe Y. Insulinoma-associated-1 (INSM1) expression in thymic squamous cell carcinoma. Virchows Archiv, 481:893-901, 2022
35. Shiraishi Y, Hakozaki T, Nomura S, Kataoka T, Tanaka K, Miura S, Sekino Y, Ando M, Horinouchi H, Ohe Y, Okamoto I. A Multicenter, Randomized Phase III Study Comparing Platinum Combination Chemotherapy Plus Pembrolizumab With Platinum Combination Chemotherapy Plus Nivolumab and Ipilimumab for Treatment-Naive Advanced Non-Small Cell Lung Cancer Without Driver Gene Alterations: JCOG2007 (NIPPON Study). Clinical lung cancer, 23:e285-e288, 2022
36. Nadal E, Horinouchi H, Shih JY, Nakagawa K, Reck M, Garon EB, Wei YF, Kollmeier J, Frimodt-Moller B, Barrett E, Lipkovich O, Visseren-Grul C, Novello S. RELAY, Ramucirumab Plus Erlotinib Versus Placebo Plus Erlotinib in Patients with Untreated, Epidermal Growth Factor Receptor Mutation-Positive, Metastatic Non-Small-Cell Lung Cancer: Safety Profile and Manageability. Drug safety, 45:45-64, 2022
37. Hibino H, Sakiyama N, Makino Y, Makihara-Ando R, Horinouchi H, Fujiwara Y, Kanda S, Goto Y, Yoshida T, Okuma Y, Shinno Y, Murakami S, Hashimoto H, Akiyoshi T, Imaoka A, Ohe Y, Yamaguchi M, Ohtani H. Evaluation of hepatic CYP3A enzyme activity using endogenous markers in lung cancer patients treated with cisplatin, dexamethasone, and aprepitant. European journal of clinical pharmacology, 78:613-621, 2022
38. Matsumoto Y, Umemura S, Okizaki A, Fujisawa D, Kobayashi N, Tanaka Y, Sasaki C, Shimizu K, Ogawa A, Kinoshita H, Uchitomi Y, Yoshiuchi K, Matsuyama Y, Morita T, Goto K, Ohe Y. Early specialized palliative care for patients with metastatic lung cancer receiving chemotherapy: a feasibility study of a nurse-led screening-triggered programme. Japanese journal of clinical oncology, 52:375-382, 2022
39. Kaku S, Horinouchi H, Watanabe H, Yonemori K, Okusaka T, Boku N, Yamazaki N, Kawai A, Ohe Y, Kusumoto M. Incidence and prognostic factors in severe drug-induced interstitial lung disease caused by antineoplastic drug therapy in the real world. Journal of cancer research and clinical oncology, 148:1737-1746, 2022
40. Okumura M, Du J, Kageyama SI, Yamashita R, Hakozaki Y, Motegi A, Hojo H, Nakamura M, Hirano Y, Okuma Y, Okuma HS, Tsuchihara K, Akimoto T. Comprehensive screening for drugs that modify radiation-induced immune responses. British journal of cancer, 126:1815-1823, 2022
41. Horinouchi H, Kusumoto M, Yatabe Y, Aokage K, Watanabe SI, Ishikura S. Lung Cancer in Japan. Journal of thoracic oncology, 17:353-361, 2022
42. Hasegawa H, Shitara K, Takiguchi S, Takiguchi N, Ito S, Kochi M, Horinouchi H, Kinoshita T, Yoshikawa T, Muro K, Nishikawa H, Suna H, Kodera Y. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric cancer, 25:619-628, 2022
43. Muto Y, Okuma Y. Therapeutic options in thymomas and thymic carcinomas. Expert review of anticancer therapy, 22:401-413, 2022
44. So C, Yoshida T, Mizuno T, Yatabe Y, Ohe Y. Rapidly progressing metastatic malignant melanoma mimicking primary pleural tumor: A case report. Thoracic cancer, 13:1423-1426, 2022
45. Ohe Y, Yamazaki N, Yamamoto N, Murakami H, Yoh K, Kitano S, Hashimoto H, Murayama A, Nakane S, Gemma A. The real-world safety of atezolizumab as second-line or later treatment in Japanese patients with non-small-cell lung cancer: a post-marketing surveillance study. Japanese journal of clinical oncology, 52:623-632, 2022
46. Otsubo K, Kishimoto J, Ando M, Kenmotsu H, Minegishi Y, Horinouchi H, Kato T, Ichihara E, Kondo M, Atagi S, Tamiya M, Ikeda S, Harada T, Takemoto S, Hayashi H, Nakatomi K, Kimura Y, Kondoh Y, Kusumoto M, Ichikado K, Yamamoto N, Nakagawa K, Nakanishi Y, Okamoto I. Nintedanib plus chemotherapy for nonsmall cell lung cancer with idiopathic pulmonary fibrosis: a randomised phase 3 trial. The European respiratory journal, 60:2200380, 2022
47. Torasawa M, Yoshida T, Yagishita S, Shimoda Y, Shirasawa M, Matsumoto Y, Masuda K, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Takahashi K, Ohe Y. Nivolumab versus pembrolizumab in previously-treated advanced non-small cell lung cancer patients: A propensity-matched real-world analysis. Lung cancer (Amsterdam, Netherlands), 167:49-57, 2022
48. Koyama T, Shimizu T, Sato J, Katsuya Y, Iwasa S, Kondo S, Yoshida T, Sudo K, Nishino M, Takiguchi Y, Yonemori K, Yamamoto N. Practical consideration for successful sequential tumor biopsies in first-in-human trials. Investigational new drugs, 40:841-849, 2022
49. Masuda K, Ishiki H, Yokomichi N, Yamaguchi T, Ito T, Takatsu H, Amano K, Hiramoto S, Yamauchi T, Kawaguchi T, Mori M, Matsuda Y, Yamaguchi T. Effect of paracentesis on the survival of patients with terminal cancer and ascites: a propensity score-weighted analysis of the East Asian Collaborative Cross-cultural Study to Elucidate the Dying Process. Supportive care in cancer, 30:6233-6241, 2022
50. Takeyasu Y, Yoshida T, Masuda K, Matsumoto Y, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Ohe Y. Lorlatinib Versus Pemetrexed-Based Chemotherapy in Patients With ALK-rearranged NSCLC Previously Treated With Alectinib. JTO clinical and research reports, 3:100311, 2022
51. Ozawa Y, Yamamoto N, Yamamoto K, Ito K, Kenmotsu H, Hayashi H, Shukuya T, Fujimoto D, Sugawara S, Niho S, Ohe Y, Okamoto H, Nakagawa K, Kiura K, Yoshino I, Gemma A. Creation of an Integrated Clinical Trial Database and Data Sharing for Conducting New Research by the Japan Lung Cancer Society. JTO clinical and research reports, 3:100317, 2022
52. Wu H, Ning J, Li Z, Divisi D, Rossi A, Cortellini A, Um SW, Okuma Y, Lazzari C, Luo Q, Chen T. Osimertinib as induction therapy for oligometastatic non-small cell lung cancer with EGFR mutation: a case report. Translational lung cancer research, 11:686-696, 2022
53. Satoh H, Arai Y, Furukawa E, Moriguchi T, Hama N, Urushidate T, Totoki Y, Kato M, Ohe Y, Yamamoto M, Shibata T. Genomic landscape of chemical-induced lung tumors under Nrf2 different expression levels. Carcinogenesis, 43:613-623, 2022
54. Kashima J, Okuma Y. Advances in biology and novel treatments of SCLC: The four-color problem in uncharted territory. Seminars in cancer biology, 86:386-395, 2022
55. Shukuya T, Takamochi K, Sakurai H, Yoh K, Hishida T, Tsuboi M, Goto Y, Kudo Y, Ohde Y, Okumura S, Taguri M, Kunitoh H. Efficacy of Adjuvant Chemotherapy With Tegafur-Uracil in Patients With Completely Resected, Node-Negative NSCLC-Real-World Data in the Era of Molecularly Targeted Agents and Immunotherapy. JTO clinical and research reports, 3:100320, 2022
56. Yang JC, Ohe Y, Chiu CH, Ou X, Cantarini M, Jänne PA, Hartmaier RJ, Ahn MJ. Osimertinib plus selumetinib in EGFR-mutated, non-small cell lung cancer after progression on EGFR-TKIs: A Phase 1b, open-label, multicenter trial (TATTON Part B). Clinical cancer research, clincanres.4329.2022-1-26 08:36:37.517, 2022
57. Shinno Y, Yoshida A, Masuda K, Matsumoto Y, Okuma Y, Yoshida T, Goto Y, Horinouchi H, Yamamoto N, Yatabe Y, Ohe Y. Efficacy of Immune Checkpoint Inhibitors in SMARCA4-Deficient Thoracic Tumor. Clinical lung cancer, 23:386-392, 2022
58. Kitadai R, Okuma Y. Treatment Strategies for Non-Small Cell Lung Cancer Harboring Common and Uncommon EGFR Mutations: Drug Sensitivity Based on Exon Classification, and Structure-Function Analysis. Cancers, 14:2519, 2022
59. Shimoda Y, Shibaki R, Yoshida T, Murakami S, Shirasawa M, Torasawa M, Matsumoto Y, Masuda K, Shinno Y, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Ohe Y, Motoi N. Concurrent High PD-L1 Expression and CD8(+) Immune Cell Infiltration Predict PD-1 Blockade Efficacy in Advanced EGFR-Mutant NSCLC Patients. Clinical lung cancer, 23:477-486, 2022
60. Yang JC, Ohe Y, Chiu CH, Ou X, Cantarini M, Jänne PA, Hartmaier RJ, Ahn MJ. Osimertinib plus Selumetinib in EGFR-Mutated Non-Small Cell Lung Cancer After Progression on EGFR-TKIs: A Phase Ib, Open-Label, Multicenter Trial (TATTON Part B). Clinical cancer research, OF1-OF10, 2022
61. Nokihara H, Kijima T, Yokoyama T, Kagamu H, Suzuki T, Mori M, Santorelli ML, Taniguchi K, Kamitani T, Irisawa M, Kanda K, Abe M, Burke T, Goto Y. Real-World Treatments and Clinical Outcomes in Advanced NSCLC without Actionable Mutations after Introduction of Immunotherapy in Japan. Cancers, 14:2846, 2022
62. Baba K, Goto Y. Lorlatinib as a treatment for ALK-positive lung cancer. Future oncology (London, England), 18:2745-2766, 2022
63. Fujii H, Okuma Y. Unarranged territory in uncommon EGFR mutations. Translational lung cancer research, 11:1233-1236, 2022
64. Zenke Y, Hakozaki T, Nakahara Y, Horinouchi H, Ohe Y. Medical management of older patients with lung cancer. Japanese journal of clinical oncology, 52:1082-1088, 2022
65. Sugawara S, Kondo M, Yokoyama T, Kumagai T, Nishio M, Goto K, Nakagawa K, Seto T, Yamamoto N, Kudou K, Asato T, Zhang P, Ohe Y. Brigatinib in Japanese patients with tyrosine kinase inhibitor-naive ALK-positive non-small cell lung cancer: first results from the phase 2 J-ALTA study. International journal of clinical oncology, 27:1828-1838, 2022
66. Kenmotsu H, Sugawara S, Watanabe Y, Saito H, Okada M, Chen-Yoshikawa TF, Ohe Y, Nishio W, Nakagawa S, Nagao H. Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010). Cancer science, 113:4327-4338, 2022
67. Goto Y, Tamura A, Matsumoto H, Isobe K, Ozaki T, Santorelli ML, Taniguchi K, Kamitani T, Irisawa M, Kanda K, Abe M, Burke T, Nokihara H. First-Line Pembrolizumab Monotherapy for Advanced NSCLC With Programmed Death-Ligand 1 Expression Greater Than or Equal to 50%: Real-World Study Including Older Patients in Japan. JTO clinical and research reports, 3:100397, 2022
68. Seki Y, Yoshida T, Kohno T, Masuda K, Okuma Y, Goto Y, Horinouchi H, Yamamoto N, Kuwano K, Ohe Y. Liquid biopsy for the detection of resistance mutations to ROS1 and RET inhibitors in non-small lung cancers: A case series study. Respiratory investigation, 60:852-856, 2022
69. Ida H, Koyama T, Mizuno T, Sunami K, Kubo T, Sudo K, Tao K, Hirata M, Yonemori K, Kato K, Okusaka T, Ohe Y, Matsui Y, Yamazaki N, Ogawa C, Kawai A, Narita Y, Esaki M, Yamamoto N. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer science, 113:4300-4310, 2022
70. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, Takeda M, Ohe Y, Khan S, Ohashi K, Soldatenkova V, Szymczak S, Sullivan L, Wright J, Drilon A. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. The Lancet. Oncology, 23:1261-1273, 2022
71. Xu F, Xiao C, Sun W, He Y, Chalela R, Masuda K, Ulivi P, Shen K, Shao Q, Xu J, Liu L. A lung adenocarcinoma patient with ROS1 fusion and NBN germline mutation achieves long progression-free survival from sintilimab combined with niraparib after failure of ROS1 inhibitors: a case report. Annals of translational medicine, 10:912, 2022
72. Ohuchi M, Yagishita S, Jo H, Akagi K, Inaba Higashiyama R, Masuda K, Shinno Y, Okuma Y, Yoshida T, Goto Y, Horinouchi H, Makino Y, Yamamoto N, Ohe Y, Hamada A. Early change in the clearance of pembrolizumab reflects the survival and therapeutic response: A population pharmacokinetic analysis in real-world non-small cell lung cancer patients. Lung cancer (Amsterdam, Netherlands), 173:35-42, 2022
73. Verschraegen C, Andric Z, Moiseenko F, Makharadze T, Shevnya S, Oleksiienko A, Yañez Ruiz E, Kim S, Ahn K, Park T, Park S, Ju H, Ohe Y. Candidate Bevacizumab Biosimilar CT-P16 versus European Union Reference Bevacizumab in Patients with Metastatic or Recurrent Non-Small Cell Lung Cancer: A Randomized Controlled Trial. BioDrugs, 36:749-760, 2022
74. Nakamura T, Takeyasu Y, Yoshida T, Ohashi K, Ohe Y. End-of-life impact of concurrent diabetes mellitus and adrenal insufficiency as immune-related adverse events in an advanced non-small cell lung cancer patient. Thoracic cancer, 13:3073-3075, 2022
75. Zhang Y, Goto Y, Yagishita S, Shinno Y, Mizuno K, Watanabe N, Yamamoto Y, Ota N, Ochiya T, Fujita Y. Machine learning-based exceptional response prediction of nivolumab monotherapy with circulating microRNAs in non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands), 173:107-115, 2022
76. Itahashi K, Irie T, Yuda J, Kumagai S, Tanegashima T, Lin YT, Watanabe S, Goto Y, Suzuki J, Aokage K, Tsuboi M, Minami Y, Ishii G, Ohe Y, Ise W, Kurosaki T, Suzuki Y, Koyama S, Nishikawa H. BATF epigenetically and transcriptionally controls the activation program of regulatory T cells in human tumors. Science immunology, 7:eabk0957, 2022
77. Yazaki S, Kojima Y, Yoshida H, Takamizawa S, Kitadai R, Nishikawa T, Shimoi T, Sudo K, Saito A, Okuma HS, Tanioka M, Noguchi E, Uno M, Ishikawa M, Kato T, Fujiwara Y, Ohe Y, Yonemori K. High expression of folate receptor alpha is associated with poor prognosis in patients with cervical cancer. Journal of gynecologic oncology, 33:e82, 2022
78. Udagawa C, Nakano MH, Yoshida T, Ohe Y, Kato K, Mushiroda T, Zembutsu H. Association between genetic variants and the risk of nivolumab-induced immune-related adverse events. Pharmacogenomics, 23:887-901, 2022
79. Terai H, Soejima K, Shimokawa A, Horinouchi H, Shimizu J, Hase T, Kanemaru R, Watanabe K, Ninomiya K, Aragane N, Yanagitani N, Sakata Y, Seike M, Fujimoto D, Kasajima M, Kubo A, Kusumoto S, Oyamada Y, Fujiwara K, Mori M, Hashimoto M, Shingyoji M, Kodani M, Sakamoto J, Agatsuma T, Kashiwabara K, Inomata M, Tachihara M, Tanaka K, Hayashihara K, Koyama N, Matsui K, Minato K, Jingu D, Sakashita H, Hara S, Naito T, Okada A, Tanahashi M, Sato Y, Asano K, Takeda T, Nakazawa K, Harada T, Shibata K, Kato T, Miyaoka E, Yoshino I, Gemma A, Mitsudomi T. Real-World Data Analysis of Pembrolizumab Monotherapy for NSCLC Using Japanese Postmarketing All-Case Surveillance Data. JTO clinical and research reports, 3:100404, 2022
80. Kohno T, Kato M, Kohsaka S, Sudo T, Tamai I, Shiraishi Y, Okuma Y, Ogasawara D, Suzuki T, Yoshida T, Mano H. C-CAT: The National Datacenter for Cancer Genomic Medicine in Japan. Cancer discovery, 12:2509-2515, 2022
81. Hakozaki T, Nolin-Lapalme A, Kogawa M, Okuma Y, Nakamura S, Moreau-Amaru D, Tamura T, Hosomi Y, Takeyama H, Richard C, Hosokawa M, Routy B. Cancer Cachexia among Patients with Advanced Non-Small-Cell Lung Cancer on Immunotherapy: An Observational Study with Exploratory Gut Microbiota Analysis. Cancers, 14:5405, 2022
82. Mazieres J, Iadeluca L, Shaw AT, Solomon BJ, Bauer TM, de Marinis F, Felip E, Goto Y, Kim DW, Mok T, Reisman A, Thurm H, Polli AM, Liu G. Patient-reported outcomes from the randomized phase 3 CROWN study of first-line lorlatinib versus crizotinib in advanced ALK-positive non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands), 174:146-156, 2022
83. Tateishi AT, Okuma Y. Onco-biome in pharmacotherapy for lung cancer: a narrative review. Translational lung cancer research, 11:2332-2345, 2022
84. Katsuya Y, Kitano S, Yamashita M, Ouchi M, Yagishita S, Hamada A, Nakamura H, Hosoda F, Shibata T, Motoi N, Nakayama T, Seto T, Umemura S, Hosomi Y, Satouchi M, Nishio M, Kozuki T, Hida T, Ohe Y, Horinouchi H. Comprehensive biomarker analysis from phase II study of nivolumab in patients with thymic carcinoma. Frontiers in oncology, 12:966527, 2022
85. Murakami H, Horinouchi H, Harada H, Sobue T, Kato T, Atagi S, Kozuki T, Tokito T, Oizumi S, Seike M, Ohashi K, Mio T, Sone T, Jinushi M, Tsuboi M. Deciphering the clinical features of heterogeneous stage III non-small cell lung cancer in Japanese real-world clinical practice: Expanded cohort of the SOLUTION study. Lung cancer (Amsterdam, Netherlands), 165:152-163, 2021
