Annual Report 2022
Department of Cellular Therapy Processing
Takahiro Fukuda, Wataru Takeda, Keiko Irie, Hidetomo Fukumoto, Ryuichi Narita, Terumichi Akiike, Tetsuro Isobe, Satoshi Hatakeyama, Kenjiro Saito, Shinpei Hiromatsu, Hidekazu Kurasawa, Tsubasa Hiratsuka, Kotomi Suse, Sayaka Takeuchi, Noriko Takahashi, Saori Nakabayashi, Yuki Kase, Shizuka Uyama, Maemi Soma
Introduction
The Department of Cellular Therapy Processing was established in 2020 as a shared department at NCCH to appropriately handle the increasing demand for cellular therapy in recent years. We support cell collection, preparation, and storage not only for hematopoietic cell transplantation (HCT) but also for new cellular therapies such as Chimeric Antigen Receptor T-cell (CAR-T) therapy.
The Team and What We Do
Our department consists of 2 physicians, 11 clinical engineers, and 6 medical technicians. In fiscal 2022, the number of cell collections was 20 for autologous peripheral blood stem cell (PBSC), 31 for related PBSC, 4 for unrelated PBSC, and 6 for unrelated bone marrow from Japan Marrow Donor Program (JDMP) donors (Table 1). We performed 30 related peripheral blood stem cell transplantations (PBSCTs) (including 20 HLA-haploidentical), 21 for unrelated PBSCTs, 2 for unrelated BMTs, 17 for cord blood transplantations (CBTs), and 22 autologous PBSCTs.
With respect to CAR-T therapy, we started full-fledged operation of Breanji® along with Kymriah®, and the number of Breanji® increased. In fiscal 2022, the number of autologous lymphocyte collections for CAR-T cells was 24, and the CAR-T therapy was performed in 21 patients.
Table 1. Number of each type of procedures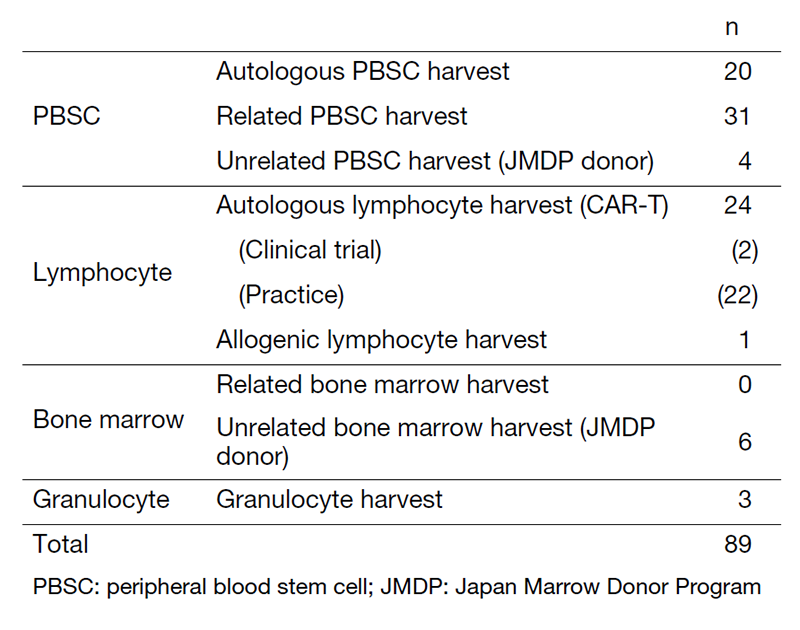

For TEMCELL®, which is an off-the-shelf bone marrow-derived mesenchymal stem cell treatment for refractory acute graft-versus-host disease (GVHD), we are responsible for the storage and preparation of cells. The number of preparations has increased in fiscal 2022 (N=179), therefore we made a substantial contribution to the treatment of severe acute GVHD.
Research Activities
We conducted a retrospective study to examine the usefulness of measuring the number of CD34-positive cells during PBSCH. We observed a correlation between the number of CD34-positive cells in the product during PBSCH (mid-apheresis product) and after PBSCH (final product). We reported that adjusting the amount of apheresis using the number of CD34-positive cells in the mid-apheresis product might enable the reliable collection of the target number of CD34-positive cells and reduce apheresis time, leading to optimal collection (Nakabayashi JSTMCT 2022).
Clinical Trials
We are involved in clinical trials of CAR-T therapies. The number of autologous lymphocyte harvests in clinical trials was 1 in the Department of Experimental Therapeutics (NIB101) and 1 in the Department of Hematology (JCAR017).
Training and Educational Activities
We have trained several certified apheresis nurses who belong to the Japanese Red Cross Society to develop their PBSC harvesting skills.
Future Prospects
Along with HCT, CAR-T therapy is expected to increase in the future. It is expected that the role of our department will increase more and more, thus we need to expand our system to provide appropriate treatment for all patients.
