Home > Information > press release > A novel biomarker for development of a new screening technique for early-stage pancreatic cancer and precancerous conditions associated with risk of pancreatic malignancy - experimental screening in Kagoshima Prefecture
A novel biomarker for development of a new screening technique for early-stage pancreatic cancer and precancerous conditions associated with risk of pancreatic malignancy - experimental screening in Kagoshima Prefecture
June 26th 2017
National Cancer Center
in Japanese
The biomarker is an isoform of apolipoprotein (apo) A2, a protein that circulates in the blood, and the NCC Research Institute has been collaborating with the National Cancer Institute (NCI) in the United States to assess biomarker effectiveness for detecting early-stage pancreatic cancer and precancerous conditions associated with a high risk of pancreatic malignancy, and to develop a test kit [../../../../jp/information/pr_release/2015/1109/index.html]. Based on the results of this study, trial screenings for pancreatic cancer were carried out in a collaborative study with Kobe University and others starting in 2015. In those trial screenings, abnormal biomarker values enabled detection of pancreatic cancer and precancerous conditions associated with a risk of pancreatic malignancy. However, sufficient data for scientific verification of biomarker utility could not be secured in the preceding study, because study participants were able to choose whether to undergo thorough examination after testing positive to the blood biomarker test, and few opted to do so.
Participants in the forthcoming study will undergo thorough examination using contrast-enhanced computed tomography (CT) after the blood biomarker test. The target for study enrollment is 5,000 to 10,000 participants.
The study will be supported by a grant for Practical Implementation of Effective Blood Biomarker-based Pancreatic Cancer Screening (Lead Scientist: Dr. Kazufumi Honda) from the Practical Research for Innovative Cancer Control program of the Japan Agency for Medical Research and Development (AMED), and the research team is drawn from the NCC, the Japan Cancer Society, the Kagoshima Prefectural Comprehensive Health Center, Kagoshima University, Kagoshima City Hospital, Izumi General Medical Center, Yokohama City University, Kobe University, Kanazawa University, and Shiga University of Medical Science.
Pancreatic cancer is an intractable cancer that is difficult to detect in the early stages. A decrease in mortality rate of pancreatic cancer can be anticipated if an effective method for screening blood samples can be established. The NCC has taken up the challenge of developing blood biomarkers (indicators) for detecting early-stage pancreatic cancer and precancerous conditions associated with a risk of pancreatic malignancy.
Outline of Experimental Pancreatic Cancer Screening
- Initiation of Screening and Screening Locations
- Community-wide health examinations conducted by the Kagoshima Prefectural Comprehensive Health Center
Makurazaki City, Kagoshima Prefecture (from July 4, 2017) and Izumi City, Kagoshima Prefecture (from mid-August 2017) - Complete medical examinations conducted by the Kagoshima
Prefectural Comprehensive Health Center in Kagoshima City (date to be determined)
- Community-wide health examinations conducted by the Kagoshima Prefectural Comprehensive Health Center
- Enrollment Target
5,000 to 10,000 participants
- Screening Periods
July 2017 to March 2019 (enrollment to be closed when target is reached)
- Clinical Study Method
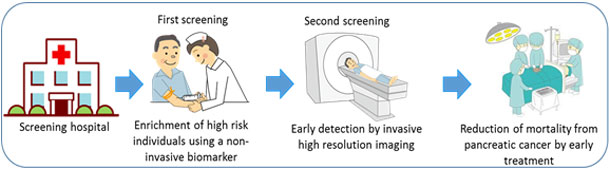
- A 7-mL volume of blood will be used for screening, and apoA2-isoform concentration will be determined at the first-stage screening.
- Study participants will be informed of the results of blood testing, and any participant with an abnormal result will undergo a thorough examination using contrast-enhanced CT as a second-stage screening. This second-stage screening will be performed at Kagoshima University, Kagoshima City Hospital, or Izumi General Medical Center as a thorough imaging examination.
- Incidences of pancreatic cancer and precancerous conditions associated with the risk of pancreatic malignancy in participants with positive blood test results (at first screening) will be determined, and the detection rate (positive predictive value) will be established.
- Cautionary Items
- Only participants undergoing a screening designated by the relevant institution in this clinical study will be enrolled. Individuals wishing to undergo biomarker testing alone will not be enrolled.
- Owing to the experimental nature of the study, the potential exists for incorrect positive results for pancreatic cancer/pre-malignancy (“false-positive result”) and incorrect negative results for pancreatic cancer/pre-malignancy (“false-negative results”). Accordingly, a positive result does not represent a certain diagnosis of cancer, and a negative result does not exclude the possibility of cancer.
Figure
Unmet Medical Needs- Pancreatic cancer is a solid tumor with very poor 5- to 10-year survival rates (4th-highest mortality rate).
Development of methods for minimally invasive early diagnosis is indispensable. - Pancreatic cancer is associated with a low morbidity rate; a screening program consistent with healthcare economics is therefore needed.
Grant support
Japan Agency for Medical Research and Development (AMED) Practical Research for Innovation Cancer Control (Kazufumi Honda.)Press release
A novel biomarker for development of a new screening technique for early-stage pancreatic cancer and precancerous conditions associated with risk of pancreatic malignancy - experimental screening in Kagoshima Prefecture (PDF:263KB)Inquiries from the media
General research inquiresKazufumi Honda
Department of Biomarker for Early Detection of Cancer
National Cancer Center Research Institute
5-1-1 Tsukiji Chuoku Tokyo 104-0045, Japan
E-mail: khonda[at]ncc.go.jp (Please replace [at] to @.)
