Home > Information > press release > An Asian Multicenter Clinical Trial Evaluating Efficacy of
Computer-Aided Detection for Colonoscopy in Colorectal Cancer Screening
An Asian Multicenter Clinical Trial Evaluating Efficacy of
Computer-Aided Detection for Colonoscopy in Colorectal Cancer Screening
January 11, 2024
National Cancer Center, Japan
in Japanese
Highlights
- To promote the early detection and treatment of colorectal cancer and its precancerous lesions in Asia, we will conduct a multicenter clinical trial to evaluate the effectiveness of colonoscopy using computer-aided detection (CADe) with artificial intelligence at 13 centers in Japan, Korea, Taiwan, Singapore, Hong Kong, and Thailand.
- This study will examine the effectiveness of colonoscopy using CADe over conventional colonoscopy without CADe in detecting lesions in colorectal cancer screening.
- In conjunction with the Asia Clinical Trials Network for Cancers Project (ATLAS project), we will develop new testing methods in Asia.
Summary
The National Cancer Center (President:Hitoshi Nakagama) Hospital (Director, Kazuaki Shimada) has initiated a clinical trial (NCCH2217, abbreviated as Project CAD) in collaboration with 13 national and international institutions to evaluate the effectiveness of computer-aided detectionNote1 (CADe) using artificial intelligence in colonoscopy.
Detecting precancerous lesions (polyps) or early cancers at the time of endoscopy is important. However, there are a certain number of missed lesions during colonoscopy procedures due to difficulty in recognition by the naked eye, anatomical blindspots, and technical skill variation among doctors. CADe utilizes artificial intelligence (AI) to detect premalignant lesions and cancers in real-time at the time of examination and assist diagnosing physicians who perform colonoscopy, which is expected to contribute to better lesion detection. In this study, we evaluate the efficacy of CADe on a large scale using medical devices that have already received regulatory and marketing approval to improve the quality of colonoscopy for colorectal cancer screening throughout Asia.
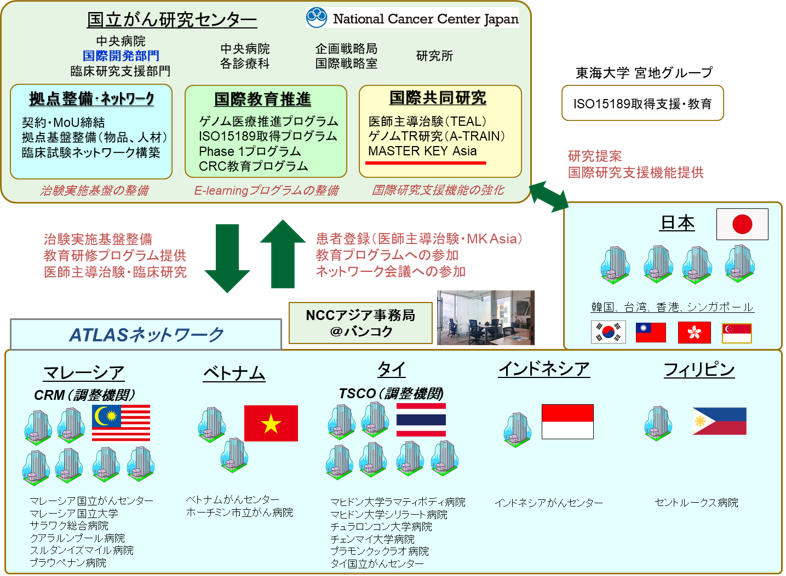
This study will be conducted in a total of 1400 examinees of colonoscopy for colorectal cancer screening (including fecal occult blood screening and primary colonoscopy screening) at 13 facilities in Asia, consisting of four facilities in Japan, the National Cancer Center Hospital, the National Cancer Center Hospital East (Kashiwa City, Chiba Prefecture), the Jikei University Hospital (Minato Ward, Tokyo), the Toho University Medical Center Omori Hospital (Ota Ward, Tokyo), and nine facilities overseas (Korea, Taiwan, Singapore, Hong Kong, and Thailand). In addition, the study is linked to the Asian Network for Clinical Trials (Asian Clinical Trials Network for Cancers Project: ATLAS project), which was launched by the National Cancer Center Hospital Note2 in 2020. The project is developing a clinical research and clinical trial network in the Asian region.
Background
Colorectal cancer is the leading cause of cancer death in many countries including Japan, and its early detection and prevention are critical to improving outcomes. Colonoscopy is known to reduce the risk of developing or dying from colorectal cancer by finding and removing precancerous lesions (polyps), in addition to detecting colorectal cancer at an early stage. However, there is a certain frequency of missed lesions during the examination, making it an important initiative to further improve the ability of colonoscopy to detect lesions.
In this context, CADe has recently gained attention as a potential tool that may help to improve the lesion-detecting ability of colonoscopy, and there are an increasing number of reports that state that CADe is helpful in colonoscopy. However, its usefulness in screening for colorectal cancer in Asia, including Japan, is yet to be underpinned.
Research methods
This study will examine the effectiveness of colonoscopy using CADe over conventional colonoscopy without CADe in detecting lesions. CADe used in this study is a medical device that has previously been developed in collaboration with a company and has already received regulatory and marketing approval. The images obtained from endoscopic devices/scopes used in routine clinical practice are indicated with lesions such as polyps and cancers, assisting physicians performing endoscopies in detecting lesions. All endoscopic devices used in the study are the same as those used in routine practice.
Study name
A multicenter randomized controlled trial to assess the usefulness of Computer-aided detection systems for colonoscopy in colorectal cancer screening in the Asia-Pacific region (research name: Project CAD)

Principle Investigator
Yutaka Saito (Director of the Endoscopy Division, National Cancer Center Hospital)
Japan Registry of Clinical Trials
jRCT number: jRCT1032230396
URL:https://jrct.niph.go.jp/latest-detail/jRCT1032230396(Link to external site.)
Colonoscopy procedures
Participants will undergo colonoscopy with either CADe colonoscopy (CADe group) or a colonoscopy without CADe (conventional group). 1:1 randomization will be used to determine subject allocation to each group. In order to minimize the risk of disadvantage of either procedure, colonoscopies will be performed by physicians that routinely perform colonoscopies for colorectal cancer screening examinees in daily clinical practice.
Study participant inclusion criteria
1) Individuals who are scheduled to undergo total colonoscopy for either reason a) or b).
a) Primary screening colonoscopy for colorectal cancer
b) Further colonoscopy due to positive fecal immunochemical test
2) Age at entry between 50 and 79 years.
3) ECOG Performance status Note3 is 0 or 1.
4) Written consent to participate in the study has been obtained.
NOTE: One may not be able to participate in this study even if meeting the above inclusion criteria.
Endpoint
The primary endpoint is colorectal adenoma detection rate (ADR). Adenomas per colonoscopy (APC) and the number of other lesions detected, the duration of endoscopy, the incidence of adverse events, etc. are set as secondary endpoints.
Planned enrollments and duration of the study
Sample size: 1,400 participants
Planned study period: 3 years (enrollment period: approximately 2 years, analysis period: approximately 1 year)
Start Date of Study: December 28, 2023
Expected End date of Study: December 31, 2026
Medical devices used in Japan
- Endoscopic Imaging Assistance Program EndoBRAIN-EYE (MAHs: Cybernet System Co., Ltd., manufacturer: Olympus Corporation, Approval Number: 30200BZX00208000)
- Endoscopy Assistance Program EW10-EC02(CAD EYE (MAHs: FUJIFILM Corporation., Approval Number: 30200BZX00288000)
- WISE VISION Endoscopic Imaging Analysis AI (MAHs: NEC Corporation, Approval Number: 30200BZX00382000)
List of participating institutions
Japan
- National Cancer Center Hospital
- National Cancer Center Hospital East
- Jikei University Hospital
- Toho University Omori Medical Center
Korea
- National Cancer Center
- Yonsei University Wonju College of Medicine
Taiwan
- National Taiwan University Hospital
- Fu Jen Catholic University Hospital
Singapore
- National University Hospital, Singapore
- Singapore General Hospital
Hong Kong
- The Chinese University of Hong Kong
- CUHK Medical Centre
Thailand
- Faculty of Medicine, Chulalongkorn University
List of research support companies
- Olympus Corporation
- FUJIFILM Corporation
- NEC Corporation
Perspective
If this study demonstrates the effectiveness of colonoscopy using CADe, it is expected that the use of CADe will become the new standard for colonoscopy in colorectal cancer screening, in turn improving the quality of colonoscopies and standardizing colorectal cancer screening practice in Asia. As such, an overall improvement in the quality of colorectal cancer screening itself can also be anticipated.
Glossary
(Note 1) CADe
CADe refers to Computer-Aided Detection. An artificial intelligence (AI) software is used to assist physicians in finding lesions by displaying the presence and location of colorectal polyps, colorectal cancers, and other lesions on an endoscopic monitor in real-time during endoscopy.
(Note 2) ATLAS project
Together with Malaysia, Thailand, the Philippines, Indonesia, and Vietnam, which are actively promoting cancer treatment development in the Asian region in the future, ATLAS project: Asian clinical TriaLs network for cAncerS project has developed a platform for international joint clinical trials led by Japan, with the aim of developing early drug development for cancer in the Asian region through the introduction of cancer genomic medicine and the implementation of physician-led clinical trials/company trials for regulatory approval applications. In addition, by promoting improvements in drug access and the full-scale introduction of cancer genomic medicine in the Asian region, we aim to increase development power across Asia and solve Asian-specific challenges in Asia.
It is expected that clinical trials will be completed at an early stage for Japan in collaboration with Asian countries, which will lead to rapid new drug approval. Ultimately, we aim to build a robust clinical trial network in the Asian region, leading Asia's development of treatments in the world.
Sep 9th, 2020 Press Release
https://www.ncc.go.jp/jp/information/pr_release/2020/0909/index.html
(Note 3) ECOG Performance status
One of the criteria set by one of the U.S. oncology organizations. (0: Fully active. 1: Physically strenuous activity is limited, but ambulatory and sedentary tasks can be performed.)
Enquiries
On Research
Masau Sekiguchi
Endoscopy Division /Cancer Screening Center, National Cancer Center Hospital
E-mail: ncch2217_office●ml.res.ncc.go.jp
Media
National Cancer Center
Office of Public Relations, Strategic Planning Bureau
E-mail: ncc-admin●ncc.go.jp

