Home > Information > press release > Press Release:Joint R&D with the Goal of Commercialization of a Scaled-Down,Methylated DNA Detection Device for Renal Cell Carcinoma Prognostication,and Other Cancers,by 2018.
Press Release:Joint R&D with the Goal of Commercialization of a Scaled-Down,Methylated DNA Detection Device for Renal Cell Carcinoma Prognostication,and Other Cancers,by 2018.
March 17, 2015
National Cancer Center
Sekisui Medical Co., Ltd.
in Japanese
National Cancer Center Japan [President: Dr. Tomomitsu Hotta, Chuo-ku, Tokyo], in conjunction with Sekisui Medical Co., Ltd. [President: Hideo Tagashira, Chuo-ku, Tokyo] has undertaken research into the prognostication of Renal Cell Carcinoma [RCC], and the commercialization of a scaled-down methylated DNA detection device. With a view to commercialization by 2018, the joint research program is now proceeding with R&D into non-RCC diagnosis devices.
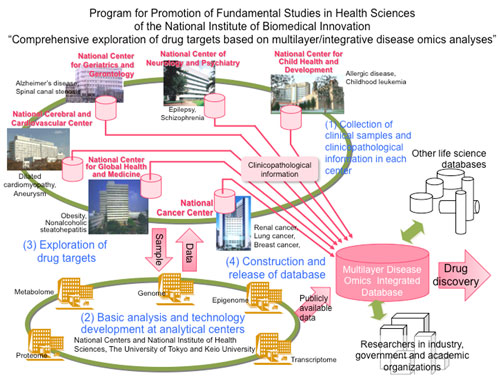
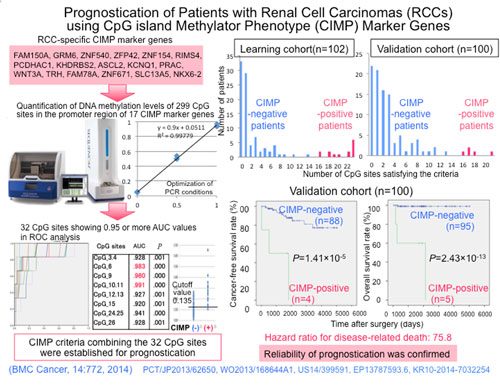
The current research results into RCC prognostication were accomplished by the National Cancer Center on behalf of the National Institute of Biomedical Innovation (President: Mr. Yoshihiro Yoneda), under the Promotion of Fundamental Studies in Health Sciences [Research Title: Comprehensive Exploration of Drug Targets Based on Multilayer/Integrative Disease Omics Analysis]. These results were published in the cancer journal BMC Cancer by this research center [Director: Dr. Hitoshi Nakagama] and its Chief of Division of Molecular Pathology; Dr. Yae Kanai.
URL: http://www.biomedcentral.com/1471-2407/14/772
Advantages of Diagnostics based on DNA Methylation
- DNA methylation not only varies depending on numerous environmental factors but is also believed to reflect the accumulated effects of the carcinogens encountered throughout the duration of one’s life. For this reason, it is possible to estimate the risk of developing cancer, as well as potentially diagnose the presence of cancer, by evaluating the methylation levels of blood or other bodily fluids and comparing this to the methylation patterns that are observed in cancerous cells.
- A clear correlation between the tumor aggressiveness/disease prognosis, and DNA methylation, is well understood. Quantifying the methylation levels helps not only in making a prognosis, but may also offer a companion diagnostic to elucidate the effectiveness of a given treatment for a number of cancers, not only RCC.
- Aberrant DNA methylation is genomically well preserved as a result of the substrate preferences of DNA methylase. Such methylation can be detected using a sufficiently sensitive method, even from a small sample volume, and for this reason it is believed that DNA methylation will prove to be an excellent biomarker.
Commercialization of scaled-down, methylated DNA detection equipment
A fast, accurate quantification of methylated DNA is essential for RCC applications in screening facilities, hospitals and clinics. However, analysis using current research is typically expensive and large-scale. Moreover, procedural complexity and costs have rendered the process unsuitable for a small number of cases, and it is not easy to use in hospitals and clinics for individual patient diagnosis.National Cancer Center & Sekisui Medical Co. Ltd. device is being developed to separate DNA fragments containing methylated-cytosine. Utilizing the polymer chemistry technology of Sekisui Medical Co. Ltd., the separation function of the HPLC column is optimized for scaled-down implementation.
Sekisui Medical Co. Ltd.’s current market entry, a HPLC Glycohemoglobin analysis device, (http://www.sekisuimedical.jp/business/diagnostics/infection/a1c/index.html
For current methylated DNA detection devices of a similar size, it is expected that for this glycohemoglobin analysis device and others, analysis should require 10-15 minutes to complete.
Outlook for the Utilization of a General-Purpose, Scaled-down Methylated DNA Detection Device.
DNA methylation may be used beyond prognostication of RCC. By assessing DNA methylation levels in blood or body fluids, the risk of carcinogenesis can be identified in previously unforeseen circumstances. Absent in normal cells, the presence of abnormal markers in methylated DNA, which occur only in cancerous cells, can potentially be used to diagnose pre-existing cancer. Quantifying the methylation levels helps not only in prognostication, but may also offer a companion diagnostic to elucidate the effectiveness of a given treatment for a number of cancers, not only RCC.Should this device we are developing be promulgated, it can be expected that while providing a foundation for diagnosis of methylated DNA, it will contribute means of providing specialized and pre-emptive medical care.
Related Patents and/or Patent Applications
-
Title: METHOD FOR JUDGING PROGNOSIS OF RENAL CELL CARCINOMA Application Number: JP 2014-044943
Filing Date: 07 Mar. 2014
Publication Date: Unpublished
Priority Data: JP 2014-039417, 28 Feb. 2014
Inventors: KANAI, Yae; ARAI, Eri; YAMADA, Yuriko; and YOTANI, Takuya. -
Title: METHOD FOR PREDICTING PROGNOSIS OF RENAL CELL CARCINOMA
International Application Number: PCT/JP2013/62650
International Filing Date: 30 Apr. 2013
International Publication Number: WO2013/168644 A1
International Publication Date: 14 Nov. 2013
National Phase Application Numbers: JP 2014-514703, US 14/399591, EP 13787593.6, CN 201380036415.8, KR 10-2014-7032254.
Priority Data: US 61/646044 11 May 2012
Inventors: KANAI, Yae; ARAI, Eri; and TIAN, Ying. -
Title: METHOD FOR ASSESSMENT OF RISK OF HEPATOCELLULAR CARCINOMA
International Application Number: PCT/JP2012/51803
International Filing Date: 27 Jan. 2012
International Publication Number: WO2012/102377 A1
International Publication Date: 02 Aug. 2012
National Phase Application Numbers: JP 2012-554863, US 13/982039.
Priority Data: JP 2011-16695, 28 Jan. 2011
Inventors: KANAI, Yae; ARAI, Eri; and NAGASHIO, Ryo.
Contact
For Further information
National Cancer Center5-1-1, Tsukiji, Chuo-Ku, Tokyo 104-0045 Japan
Chief, Division of Moleculer Pathology,
National Cancer Center Research Institute
Yae KANAI
Tel : +81-3-3542-2511
FAX: +81-3-3248-2463
E-mail: ykanai[at]ncc.go.jp (Please replace [at] to @.)
Sekisui Medical Co., Ltd.
3-3-1, Koyodai, Ryugasaki, Ibaraki 301-0852 Japan
Tsukuba Research Institute
Research & Development Division
Takuya YOTANI
Tel : +81-297-62-6425
FAX: +81-297-62-8635
E-mail: yotani002[at]sekisui.com (Please replace [at] to @.)
Other information
National Cancer Center5-1-1, Tsukiji, Chuo-Ku, Tokyo 104-0045 Japan
Office of Public Relations
Tel : +81-3-3542-2511
FAX: +81-3-3542-2545
E-mail: ncc-admin[at]ncc.go.jp (Please replace [at] to @.)
Sekisui Medical Co., Ltd.
13-5, Nihombashi 3-chome, Chuo-ku, Tokyo General Affairs
FAX:+81-3-3278-8774