Home > Information > press release > Large-scale genome sequencing of biliary tract cancer
Large-scale genome sequencing of biliary tract cancer
August 11, 2015
National Cancer Center
in Japanese
Key findings
- The comprehensive identification of driver genes including new ones was revealed by the world's largest biliary tract cancer genome sequencing project.
- Genomic aberrations to serve as potential therapeutic targets were detected in approximately 40% of biliary tract cancer patients.
- Biliary tract cancer patients likely to respond to the immune checkpoint therapy were identified.
National Cancer Center (President: Tomomitsu Hotta, Tokyo) performed large-scale genome (DNA) and transcriptome (RNA) sequencing of biliary tract cancer to elucidate novel genomic aberrations as candidate therapeutic targets and to describe the characteristics of these aberrations at each site of occurrence (intra- and extrahepatic bile ducts and gall bladder). Also, the potential effectiveness of the immune checkpoint therapy in a poor prognosis group, as identified by gene expression data, was demonstrated.
This research was conducted as a part of the international cancer genome project, the “International Cancer Genome Consortium” (“ICGC”, URL: www.icgc.org), by a research group from the Division of Cancer Genomics (Chief: Tatsuhiro Shibata) with the support of the “Practical Research for Innovative Cancer Control” by the Ministry of Health, Labour and Welfare of Japan and, since 2015, by the Japan Agency for Medical Research and Development (AMED). The results of this study were published in the international scientific journal, Nature Genetics (online version) in August 10, 2015 (GMT).
URL: http://www.nature.com/ng/journal/vaop/ncurrent/full/ng.3375.html
Video: Genomic spectra of biliary tract cancer
Biliary tract cancer (BTC), including intra-hepatic (ICC) and extra-hepatic (ECC) cholangiocarcinomas and gallbladder cancer, is a rare cancer. The incidence has increased globally; however, no effective targeted molecular therapies have been approved at the present time.
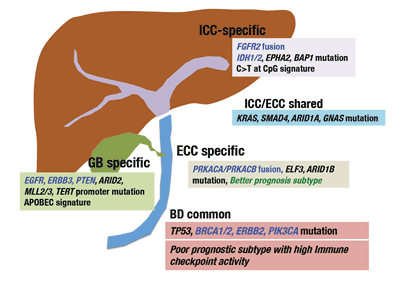
The aims of this research are to identify therapeutic targets for biliary tract cancer and to develop novel therapies based on these targets in the world’s largest genome (DNA) and transcriptome (RNA) analyses performed to date. Genomic (DNA) and transcriptomic (RNA) sequencing analyses were performed using clinical specimens collected from 260 patients (145 cases of intrahepatic bile duct cancer, 86 cases of extrahepatic bile duct cancer, and 29 cases of gallbladder cancer), which revealed detailed characteristics of genomic aberrations in biliary tract cancer, leading to the identification of 32 critical driver genes. As shown in the figure, many driver genes specific to the intra- and extrahepatic bile ducts and gall bladder were found to be involved in carcinogenesis.
The identified genomic aberrations in biliary tract cancer included at least 14 candidate therapeutic target genes (therapeutic agents targeting these genes are already under clinical development). Approximately 40% of the tumors carried at least one of these genomic aberrations.
Immune checkpoints are mechanisms through which cancer cells are known to evade an attack via the suppression of host immune cell function. Immune checkpoint inhibitors have recently attracted global attention as therapeutic agents against cancer. The effectiveness of several such inhibitors has already been demonstrated against melanoma (a type of skin cancer) and lung cancer in large-scale clinical trials, and they are currently being energetically investigated for use against other types of cancer. Our results suggest that some biliary tract cancer patients respond to immune checkpoint inhibitors. The continued development of immune checkpoint inhibitors is expected to result in the development of effective treatment options against biliary tract cancer.
Prospects
We will focus on the following three points to improve the efficacy of biliary tract cancer treatments;- Launch “All Japan” (nationwide) gene screening and umbrella clinical trials using the actionable driver gene sets identified in this research as potential therapeutic targets for the treatment of Japanese biliary tract cancer patients.
- Promote the development of treatments based on these newly discovered driver genes.
- Accelerate the development of immunotherapies including immune checkpoint inhibitors because of the potential effectiveness, initially demonstrated against biliary tract cancer in this study.
Reference
Hiromi Nakamura, Yasuhito Arai, Yasushi Totoki, Tomoki Shirota, Asmaa Elzawahry, Mamoru Kato, Natsuko Hama, Fumie Hosoda, Tomoko Urushidate, Shoko Ohashi, Nobuyoshi Hiraoka, Hidenori Ojima, Kazuaki Shimada, Takuji Okusaka, Tomoo Kosuge, Shinichi Miyagawa, and Tatsuhiro Shibata*, “Genomic spectra of biliary tract cancerVideo: Genomic spectra of biliary tract cancer
About National Cancer Center
Since establishment in 1962 by Ministry of Health and Welfare, National Cancer Center (“NCC”) is the Japanese government’s agency for cancer research, patient care, and training, in coordination with the national cancer control program under the auspices of Basic Law for Anticancer Measures.
URL: http://www.ncc.go.jp/en/index.html
Media Contacts
Dr. Tatsuhiro Shibata, M.D., Ph.D.Chief, Division of Cancer Genomics
National Cancer Center Laboratory of Molecular Medicine, Human Genome Center, The institute of Medical Science, the University of Tokyo
Tel: +81-3-3542-2511 (Ext. 3123)
FAX: +81-3-3547-5137
E-mail: tashibat[at]ncc.go.jp (Please replace [at] to @.)
Office of Public Relations, Strategic Planning Bureau
National Cancer Center
Tel: +81-3-3542-2511 (Main)
FAX: +81-3-3542-2545
E-mail: ncc-admin[at]ncc.go.jp (Please replace [at] to @.)