Home > Information > press release > The development of a plasma biomarker for early detection of pancreatic cancer
The development of a plasma biomarker for early detection of pancreatic cancer
November 9, 2015
National Cancer Center
in Japanese
- National Cancer Center (“NCC”, President: Dr. Tomomitsu Hotta, Location: Tokyo Japan) discovered that apolipoprotein A2 (apoA2) isoforms in the blood were significantly decreased in early stage pancreatic cancer and in risk diseases of pancreatic cancer compared to normal, healthy controls.
- Dr. Kazufumi Honda and his team recently established a plasma biomarker for early detection of pancreatic cancer using ELISA measurement of apoA2-isoforms. Its clinical applicability was confirmed by collaboration with a Japanese multi-institutional study and the National Cancer Institute Early Detection Research Network (NCI EDRN, Chief Dr. Sudhir Srivastava, MD USA).
- In diagnostic accuracy testing to distinguish patients with pancreatic cancer from healthy controls, apoA2 isoforms showed higher accuracy than that of an existing plasma biomarker for pancreatic cancer (CA19-9).
- To apply this biomarker to clinical practice, the researchers have started experimental pancreatic cancer screening using this biomarker, with the support of the Japan Agency for Medical Research and Development (AMED).
Publication of this study
This research was conducted by Dr. Kazufumi Honda of National Cancer Center Research Institute (NCCRI, Director Dr. Hitoshi Nakagama, Tokyo Japan) and his team, and these findings were published in Scientific Reports (http://www.nature.com/srep/) on the 9th November. Their aim is to develop an in vitro diagnostic biomarker to screen patients with early stage pancreatic cancer and risk diseases of pancreatic cancer. This study was supported by the Japan Agency for Medical Research and Development (AMED).Abstract of this study
Pancreatic cancer is one of the most lethal solid malignant tumors. To decrease the mortality of pancreatic cancer, efficient screening methods that would enable detection of the disease at an early stage and the identification of pancreatic lesions that are thought to be risk factors for pancreatic malignancy are needed. Plasma/serum biomarkers for the early detection of pancreatic cancer would be useful for screening to identify those patients who should undergo a second screening using stricter diagnostic modalities that can detect pancreatic dysfunction before imaging. The team reported the development of novel enzyme-linked immunosorbent assays (ELISAs) for measurement of apoA2 isoforms and their clinical applicability for early detection of pancreatic cancer. Plasma and serum concentrations of apoA2 isoforms were measured in three independent cohorts, which comprised healthy control subjects and patients with pancreatic cancer and gastroenterologic diseases (n=1156). These cohorts included 151 cases of stage I/II pancreatic cancer which were collected by Japanese medical institutions and NCI EDRN. ApoA2 isoform levels not only distinguished the early stages of pancreatic cancer from healthy controls but also identified patients at high risk for pancreatic malignancy. The diagnostic accuracy of apoA2 isoforms for distinguishing early stage pancreatic cancer from healthy controls was higher than that of the existing plasma biomarker for pancreatic cancer, CA19-9, in all independent cohorts.The level of ApoA2 isoforms is a potential biomarker for screening patients for the early stage of pancreatic cancer and for identifying patients at risk for pancreatic malignancy.Figure 1. Distribution and Receiver Operating Characteristic analysis (ROC) for ELISA of an apoA2 isoform in a Japanese multi-institutional study.
IELISA assay detected a significant decrease in the level of the apoA2 isoform, ApoA2-ATQ/AT in stage-I, stage-II, stage-III and stage-IV of invasive ductal adenocarcinoma of the pancreas (IDACP) in comparison with healthy controls. ELISA assay of the level of this apoA2 isoform showed high diagnostic accuracy for the detection of patients with IDACP.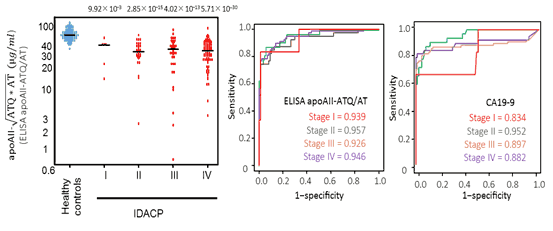
Figure 2. Distribution of an apoA2 isoform in the pancreatic cancer reference set of NCI EDRN.
A significant reduction in the level of the apoA2 isoform was found in pancreatic cancer in comparison with healthy controls.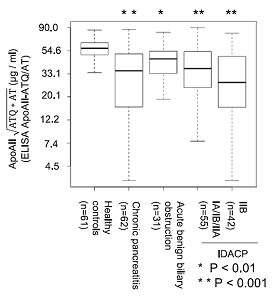
| Journal Name | Scientific Reports |
|---|---|
| Title | Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein-AII isoforms |
| Authors | Kazufumi Honda* (*correspondence author), Michimoto Kobayashi, Takuji Okusaka, Jo Ann Rinaudo, Ying Huang, Tracey Marsh, Mitsuaki Sanada, Yoshiyuki Sasajima, Shoji Nakamori, Masashi Shimahara, Takaaki Ueno, Akihiko Tsuchida, Naohiro Sata, Tatsuya Ioka, Yohichi Yasunami, Tomoo Kosuge, Nami Miura, Masahiro Kamita, Takako Sakamoto, Hirokazu Shoji, Giman Jung, Sudhir Srivastava & Tesshi Yamada |
| Doi | 10.1038/srep15921 |
Support Grants
This work was supported by a Japan Agency for Medical Research and Development (AMED) Practical Research for Innovation Cancer Control (15ck0106101h0002), Applied Research for Innovative Treatment of Cancer grant from the Ministry of Health, Labour and Welfare of Japan, AMED P-direct, AMED-CREST.Press release
The development of a plasma biomarker for early detection of pancreatic cancer(PDF:215KB)Contact
Dr. Kazufumi HondaLaboratory Head
Division of Chemotherapy and Clinical Research
National Cancer Center Research Institute
5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Email: khonda[at]ncc.go.jp (Please replace [at] to @.)
Media contact
Office of Public RelationsNational Cancer Center
5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045
E-mail:ncc-admin[at]ncc.go.jp (Please replace [at] to @.)
