Home > Information > press release > The MASTER KEY PROJECT
Genomic medicine in Rare Cancers:
A collaboration between the industry and academia
The MASTER KEY PROJECT
Genomic medicine in Rare Cancers:
A collaboration between the industry and academia
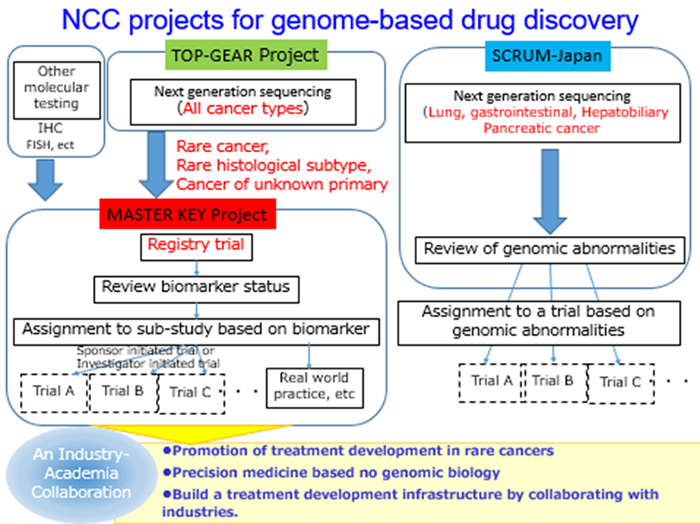
The "MASTER KEY Project" is an industry-academic collaborative project promoting rare cancers research and development and genomic medicine led by National Cancer Center Hospital (NCCH; director: Toshiro Nishida) and the NCC Research institute.
Rare cancers are cancers with a low incidence (annual incidence <6 per 100,000 patients), which makes it difficult to conduct research at and clinical trials due to the limited amount of available clinical data from patients from each individual rare cancer. The "MASTER KEY Project" is the first attempt in the world to collaborate between NCC and pharmaceutical companies to promote genomic medicine in rare cancers.
The "MASTER KEY Project" consists of two major sections. The first is a registry study that comprehensively collects genetic information, clinical information, prognostic data, etc. of patients with rare cancers and constructs a large-scale database (registry part). Data is shared with participating companies and is used for biomarker discovery and drug development.
The second section represents the implementation of a new generation clinical trial called “basket trial” (sub-study part). Assignment of patients to investigator-initiated or sponsor-initiated clinical trials will be based on their specific types of biomarkers (genetic abnormality / protein expression, etc.) regardless of the cancer location. NCCH is currently collaborating and sharing results with 11 pharmaceutical companies, that agreed to provide investigational drugs and joint research funds.
The registry study has started from May 2017, with a goal of 100 cases registered per year (250 cases so far). As for the sub-study clinical trials, respectively 7 and 5 sub-studies are ongoing or in preparation as of August 2018. Kyoto University Hospital has also joined as the first collaborating research facility in western Japan, as of April 2018, to expand the implementation system throughout Japan.
Background
Rare cancers are defined as "malignant tumors with an incidence of less than 6 per 100,000 people per year" in Europe and "malignant tumors that occur in fewer than 15 out of 100,000 people each year" in the United States. In Japan, in March 2015, the "Study Group on Rare Cancer Medicine and Support", appointed by the Ministry of Health, Labor and Welfare’s, established a definition of rare as follows: (1) “malignant tumors with an incidence of less than 6 per 100,000 people per year” and (2) “rare histological subtypes of major cancers”.
As the number of patients with rare cancers is limited, pharmaceutical companies have not necessarily actively developed anticancer drugs against rare cancers.
To overcome this issue, we at NCC, believe that NCC plays a critical role in establishing a vital network of diagnosis and treatment for rare cancer patients, as well as addressing the challenges and making new proposals to promote research and treatment development for rare cancers.
In June 2014, we established the "Rare Cancer Center" to actively engage in the research and treatment of rare cancers. Nowadays, at NCCH, we are offering clinical practice of high quality and numerous clinical trial to 1,000 ~ 1,500 rare cancer patients a year. In addition, in November 2015, NCCH established a genetic testing room compliant with CLIA * 1, with US clinical laboratory quality assurance standards and is currently working on the implementation of genomic treatment of cancer through the "TOP-GEAR project".
As a result of such efforts, the aggregation of diagnostic and research of rare cancers and the construction of a genetic testing system have progressed, and in order to further promote research, treatment development, and genomic medicine in the future, we launched the industry-academic collaborating "MASTER KEY Project".
Overview of the Master Key Project
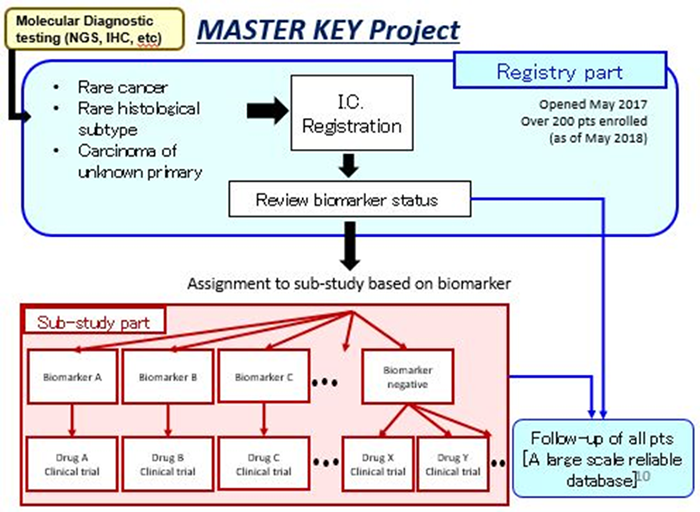
Registry part: Eligibility Criteria
Rare cancers patients undergoing medical treatment at NCCH, are eligible based on their biomarker information (genetic abnormality / protein expression), and on the following criteria:
- Rare cancers (incidence less than 6 per 100,000 people per year)
- Cancers of unknown primary
- Rare histological subtypes (incidence less than 6 per 100,000 people per year) of major cancers (stomach cancer, colon cancer, lung cancer, breast cancer, liver cancer, etc)
Clinical trial (sub-study) part
- Based on biomarker information (genetic abnormality / protein expression, etc) from the registry study, patients will be enrolled to individualized clinical trials (sub-studies) with specific molecular targeted drugs.
- In the case where the patient has no specific biomarker, a drug expected to be effective on a wider range of patients, regardless of a biomarker, will be used
- Translational research will be conducted using specimens from clinical trials in cooperation with laboratories
Collaborating industries (as of Jun-2018)
- Astellas
- Chugai
- Daiichi Sankyo
- Eisai
- Ignyta
- Kyorin
- Novartis
- Ono Pharmaceuticals
- Pfizer
- Taiho Pharmaceuticals
- Takeda
GLOSSARY
*1 CLIA(Clinical Laboratory Improvement Amendment): amendment including quality control of clinical laboratories by the US clinical laboratory improvement method. Laboratories that have received the certification are required to maintain quality through periodic inspections and guarantee quality control in inspection.
Contact
MASTER KEY Project Registry / clinical trial
Research Management Division, National Cancer Center Hospital, Tokyo
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045
E-mail:NCCH1612_office●ml.res.ncc.go.jp(Please replace ● with @)
Others
Office of Public Relations, Strategic Planning Bureau
E-mail:ncc-admin●ncc.go.jp(Please replace ● with @)
