Home > Information > press release > Asian Early Phase Oncology Drug Development Consortium Formed
Asian Early Phase Oncology Drug Development Consortium Formed
November 27, 2017
National Cancer Center
in Japanese
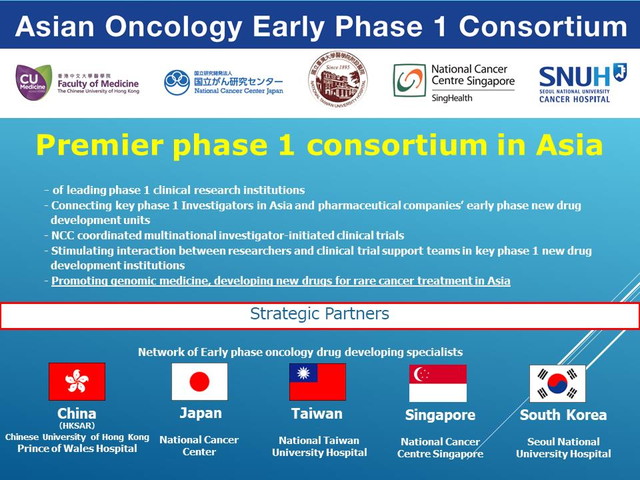
The National Cancer Center (NCC) of Japan signed a memorandum of understanding with early phase new drug development institutions (phase 1 centers) in China, South Korea, Singapore and Taiwan, to form the Asian Oncology Early Phase 1 Consortium (the Consortium), to drive the momentum towards international collaborative phase 1 clinical trials across Asia, and to realize efficient clinical development of early phase oncology drugs serving the region.
Following the conclusion of the agreement on September 14, the Consortium’s practitioners from each member country again convened on November 19 to discuss specific plans for implementing phase 1 clinical trials starting in early 2018, and strategies for the Consortium.
By promoting multinational collaborative clinical trials across Asia initiated by pharmaceutical companies with this platform, multinational investigator-initiated clinical trials coordinated by NCC investigators, collaboration with early phase drug development divisions of pharmaceutical companies, and personnel exchange between member institutions, NCC aims to advance early phase drug development in Asia, in particular genomic medicine and treatments for rare cancers.
Yasuhiro Fujiwara, Director-General of the Strategic Planning Bureau, NCC, and Deputy Director of NCC Hospital, commented that Asia needs to take the initiative in developing drugs to treat cancers prevalent in the region, namely those of the stomach, liver, and biliary tract. Drug discovery efforts initiated in Asia, rather than traditional developers of North America and Europe, are needed to develop treatments suitable for Asian populations. Fujiwara believes that international collaborative clinical trials by a premier group of leading institutions of Asian countries will play a significant role.
Member Institutions
- Japan
National Cancer Center, Japan - China
Faculty of Medicine, Chinese University of Hong Kong
Prince of Wales Hospital - Taiwan
National Taiwan University Hospital - Singapore
National Cancer Centre Singapore - South Korea
Seoul National University Hospital
Outline of Collaboration
- Collaborative framework of leading early phase new drug development institutions in Asia
- Opportunities for international collaborative clinical trials (both industry-sponsored and investigator-initiated) shared among member institutions, in early phase clinical studies (phase 1), and in collaborative research in Asia
- Collective, coordinated effort inviting pharmaceutical companies to utilize the Consortium
Outlook
- Advancement of international collaborative clinical trials of early phase new drug development in Asia initiated by pharmaceutical companies, promoted by Consortium platform
- Collaborative investigator-initiated clinical trials and research initiated by NCC investigators
- Strengthened partnership with early phase new drug development divisions of pharmaceutical companies and major early phase new drug development institutions (phase 1 centers) in Asia
- Comprehensive development of Consortium, to bridge early and late phase oncology drug development in Asia (Coordinated pan-East Asian clinical trials)
- Focus on promoting genomic medicine, and rare cancer drug development
- Closely coordinated monitoring of latest developments in clinical trial pharmaceutical regulations, and in ethics committees in each Asian country
- Interaction promoted between young researchers, support staff of premier early phase new drug development institutions in Asia
- Improvement in citizens’ health and welfare, development of industries, through new drug development in East Asia
Consortium Prospects

Consortium key investigators with the signed documents
Media inquiries
About the Memorandum of Understanding
Toshio Shimizu, MD., Head of Physician (Oncology Phase 1 Unit), Department of Experimental Therapeutics
National Cancer Center Hospital
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045
Phone: 03-3542-2511
Other (general information)
Office of Public Relations, Strategic Planning Bureau, National Cancer Center
Phone:03-3542-2511
FAX:03-3542-2545
E-mail:ncc-admin●ncc.go.jp (Replace ● to @)
