Home > Information > press release > A secondary RET mutation in the activation loop conferring
resistance to vandetanib through allosteric effects
A secondary RET mutation in the activation loop conferring
resistance to vandetanib through allosteric effects
-Elucidation of a novel drug resistance mechanism based on
a nationwide genome screening program LC-SCRUM-Japan-
February 14, 2018
National Cancer Center
Kyoto University
RIKEN
The University of Tokyo
The Francis Crick Institute
Japan Agency for Medical Research and Development
in Japanese
TOKYO, Japan (February 14, 2018) –The National Cancer Center, Kyoto University, RIKEN, the Francis Crick Institute and other institutions today announced elucidation of a novel mechanism underlying acquired resistance to RET tyrosine kinase inhibitor (TKI) in lung cancer.
Lung adenocarcinoma is the most common type of lung cancer worldwide, with incidence and mortality rates increasing in both Asian and Western countries. Oncogenic fusions of the RET kinase gene are present in 1‒2% of LADCs. RET fusion is a target for the treatment using clinically active RET tyrosine kinase inhibitors (TKIs) such as vandetanib. However, the mechanisms underlying acquired resistance to RET TKIs in lung cancer patients had been unknown.
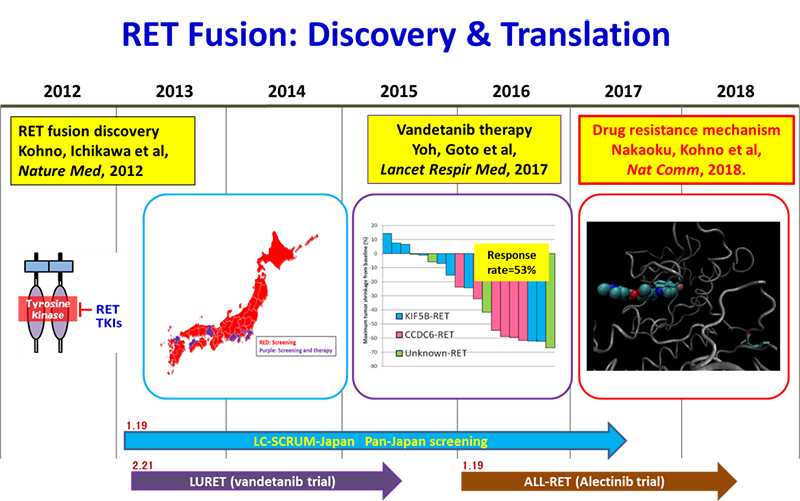
Here, we report the first case of a secondary RET mutation associated with resistance to the RET TKI vandetanib. The patient described was enrolled into our clinical trial, LURET. In this trial, 19 RET fusion-positive cases were enrolled through genetic screening by LC-SCRUM-Japan of 1,536 patients, and 17 eligible cases showed a response rate of 53% and a progression-free survival period of 4.7 months.
Resistance to vandetanib, a type I RET kinase inhibitor, developed in a patient with metastatic LADCs with RET fusion that initially exhibited a response to treatment. The resistant tumor acquired a secondary mutation resulting in a serine-to-phenylalanine substitution at codon 904 in the activation loop of the RET kinase domain.
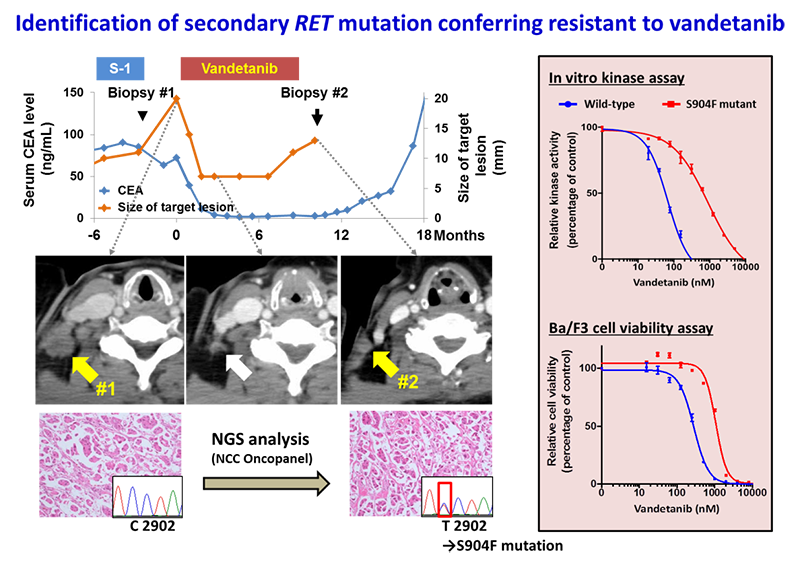
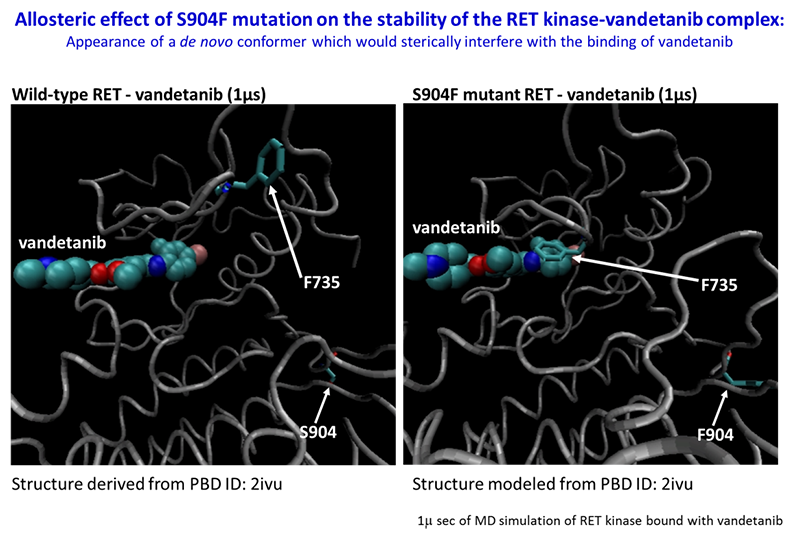
The S904F mutation confers resistance to vandetanib by increasing the ATP affinity and autophosphorylation activity of RET kinase. A reduced interaction with drug is also observed for the S904F mutant by thermal shift assay, supported by molecular dynamics simulation. A crystal structure of the S904F mutant reveals a small hydrophobic core around F904 likely to enhance basal kinase activity by stabilizing an active conformer. Our findings indicate that missense mutations in the activation loop of the kinase domain are able to increase kinase activity and confer drug resistance through allosteric effects.
RET fusion
Oncogenic fusions of the RET kinase gene is a driver oncogene alteration present in 1‒2% of LADCs. These fusions are promising targets for the treatment of LADC because of the availability of clinically active RET TKIs such as vandetanib and cabozantinib.
KIF5B-RET fusions in lung adenocarcinoma. Kohno T*, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, Iwakawa R, Ogiwara H, Oike T, Enari M, Schetter AJ, Okayama H, Haugen A, Skaug V, Chiku S, Yamanaka I, Arai Y, Watanabe S, Sekine I, Ogawa S, Harris CC, Tsuda H, Yoshida T, Yokota J, Shibata T. Nat Med. 2012 Feb 12;18(3):375-7. doi: 10.1038/nm.2644.
LURET study
Lung Cancer with RET Rearrangement Study (clinical trial registration number: UMIN000010095, see https://upload.umin.ac.jp/). This study investigated the efficacy of vandetanib for the treatment of lung cancer with RET fusion in Japan.
Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K*. Lancet Respir Med. 2017 Jan;5(1):42-50. doi: 10.1016/S2213-2600(16)30322-8.
LC-SCRUM-Japan
In February 2013, research, governmental, and pharmaceutical agencies in Japan initiated a nationwide genome screening program (LC-SCRUM-Japan) as a clinical research to detect multiple oncogene alterations, including RET and ROS1 fusions and BRAF mutation, in lung cancer patients. As of December 2017, more than 5,000 patients from 251 institutions in Japan had been enrolled.
Patients who were positive for those oncogene alterations have been receiving (or received) targeted therapies using investigational drugs in clinical trials according to their gene alterations.
Publication
- Journal
Nature Communications, 2018, on line publication. - Title
A secondary RET mutation in the activation loop conferring resistance to vandetanib. - Authors
Takashi Nakaoku, Takashi Kohno*, Mitsugu Araki, Seiji Niho, Rakhee Chauhan, Phillip P. Knowles, Katsuya Tsuchihara, Shingo Matsumoto, Yoko Shimada, Sachiyo Mimaki, Genichiro Ishii, Hitoshi Ichikawa, Satoru Nagatoishi, Kouhei Tsumoto, Yasushi Okuno, Kiyotaka Yoh, Neil Q. McDonald, Koichi Goto. (*corresponding author) - DOI
10.1038/s41467-018-02994-7 - URL
https://www.nature.com/articles/s41467-018-02994-7
Research Funding
This work was supported in part by grants-in-aid from the Japan Agency for Medical Research and Development (AMED) (JP17ck0106255, JP17ck0106148, and JP17ak0101067) and the National Cancer Center Research and Development Fund. The simulation study was supported by the FOCUS Establishing Supercomputing Center of Excellence, JST, CREST “Big data application” and MEXT, as “Priority Issue 1 on Post-K computer” (Building Innovative Drug Discovery Infrastructure through Functional Control of Biomolecular Systems). This research used computational resources of the K computer provided by the RIKEN Advanced Institute for Computational Science through the High Performance Computing Infrastructure System Research Project (Project ID: hp150272 and hp160213). This work was also supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001115), the UK Medical Research Council (FC001115) and the Wellcome Trust (FC001115); by the NCI/NIH (grant reference 5R01CA197178); by the Association for Multiple Endocrine Neoplasia Disorders MTC Research Fund.
Press release
A secondary RET mutation in the activation loop conferring resistance to vandetanib (PDF)
For research and development inquiries
National Cancer Center Research Institute
Division of Genome Biology
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Telephone: +81-3-3547-5272
FAX: +81-3-3542-0807
For media inquiries
National Cancer Center
Office of Public Relations, Strategic Planning Bureau
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
Telephone:+81-3-3542-2511
FAX:+81-3-3542-2545
E-mail: ncc-admin@ncc.go.jp
