Home > Information > press release > National Cancer Center Hospital offers Next Generation Sequencing tests as ‘Advanced Medical Care’
Towards coverage under national health insurance system
National Cancer Center Hospital offers Next Generation Sequencing tests as ‘Advanced Medical Care’
Towards coverage under national health insurance system
April 3, 2018
National Cancer Center of Japan
in Japanese
National Cancer Center Hospital is to provide tests with comprehensive analyses of cancer related gene mutations to support decision making of treatments for individual patients, effective April 9. The test, designated as an ‘Advanced Medical Care’ program, is a companion diagnostic that can be delivered to patients in combination with clinical care covered by statutory and universal national health insurance schemes (UMIN000032166).
The assessment, developed with ‘NCC Oncopanel’ can detect genetic mutations in 114 genes including 12 fusions specific to Japanese cancer patients with a single test. The diagnostic laboratory test itself, as well as the developed data analysis software, hospital support units, and expert panels to scrutinize treatment options, have been validated during the last five years at the Hospital under the research program ‘TOP-GEAR project’ (UMIN000011141). This comprehensive genomic in vitro companion diagnostic is now being applied for approval from Japanese authorities (MHLW and PMDA) for eventual coverage under the national healthcare insurance scheme, in collaboration with Sysmex Corporation.
‘Advanced Medical Care’ is a governmental scheme introduced as a means to assess the efficacy and toxicity of new treatments/devices on a clinical trial basis, for future inclusion into mainstream healthcare, with full health insurance coverage. The assessment of this diagnostic is titled “Research on Multiple Gene Panel Testing to Advance Personalized Medicine”.
This is offered to patients over 16 years of age with no standard treatments, or with no further treatments available for either solid or non-primary tumors. Together with other designated core hospitals for cancer genomic medicine or cooperative hospitals for cancer genomic medicine across Japan, 205 to 350 patients are to be enrolled in this experimental scheme to prove the efficacy of NCC Oncopanel diagnosis system. The results will also help to identify candidate patients for industry sponsored/investigator initiated cancer clinical trials, paving the way to research for new drugs offered under the national healthcare insurance scheme.
The total cost of sequencing about JPY 670,000 will be reduced with research fund subsidies, resulting in a bill of JPY 470,000 plus additional fees of approximately JPY 25,000 for relative consultation / screening / administrative fees to the patient.
Background
With advances in genomics, various genetic mutations causing cancer are discovered. Some cancers with specific genetic mutations respond extremely well to corresponding targeted drugs. Thus, targeted therapy of breast cancer with amplified HER-2 genes, of non-small cell lung cancer with EGFR mutation and ALK fusion genes, and of malignant melanoma with BRAF mutation, are offered with national health insurance scheme coverage in Japan. The current standard molecular genetic tests target one alteration each.
The more recently developed next generation sequencing allow parallel profiling of genes, enabling the comprehensive detection of gene mutations with drastically improved efficiency. Stratifying and treating patients by tumor genomic profiles has proven effective, irrelevant of primary tumor sites. Thus cancer patients without standard treatment options, or patients who have already exhausted those may benefit significantly from the comprehensive genomic assays of tumor tissue.
enomic medicine for cancer, enabling individualized treatment corresponding to gene mutations of cancer tissues, is central to the Third Basic Plan to Promote Cancer Control Programs of the Japanese Government, aiming to establish a system in which cancer genomic medicine will be available in all corners of the country.
Genetic testing by “NCC Oncopanel”
Through this genetic test, one single test allows to investigate many cancer related genetic mutations, a powerful means for selection of effective anticancer drugs. On the other hand, the test may not be of any help if no mutations are found, or when identified mutations are non-actionable. So far, the percentage of patients who were administered drugs with newly found directions from the test was about 10%. Please keep in mind the above before any decisions are made, and consult your physician, also evaluating the costs and candidate qualifications.
Patients requirements for this study
- over 16 years old
- Overall good physical condition
- Diagnosed with malignant solid tumor (solid cancer *) by pathological diagnosis
- Tumor of (1) or (2) with unresectable or recurrent lesion
(1)Solitary cancer with no standard treatment, standard treatment completed, or expected to end
(2)cancer of unknown origin **
*Solid tumor
Abnormal mass of tissue (eg, stomach cancer, lung cancer, breast cancer, etc.) without cysts or liquid areas. Leukemia and lymphoma do not form solid tumors
**Cancer of unknown primary origin
Type of cancer that developed at another site (metastatic lesion) than the organ where the cancer occurred (primary site), though the origins are unknown. It accounts for 3 to 5% of all solid tumors.
Method of research
- Informed consent process will be conducted as part of advanced medical care
- Tumor tissues will be prepared for genetic analysis, then evaluated whether the analysis can be conducted
- Blood will be drawn for comparison against tumor tissue
- DNA will be extracted from tumor tissue and blood samples, to be analyzed through NCC oncopanel
- The comprehensive mutation analysis will be evaluated by a an expert panel, a committee composed of several experts in the hospital or partner hospitals
- The results of the gene analysis will be returned to the physician in charge
- The results of the gene analysis will be explained to the patient by the physician in charge
Duration of the analysis
The period between examination and results to be returned to the patient is expected to be slightly less than a month.
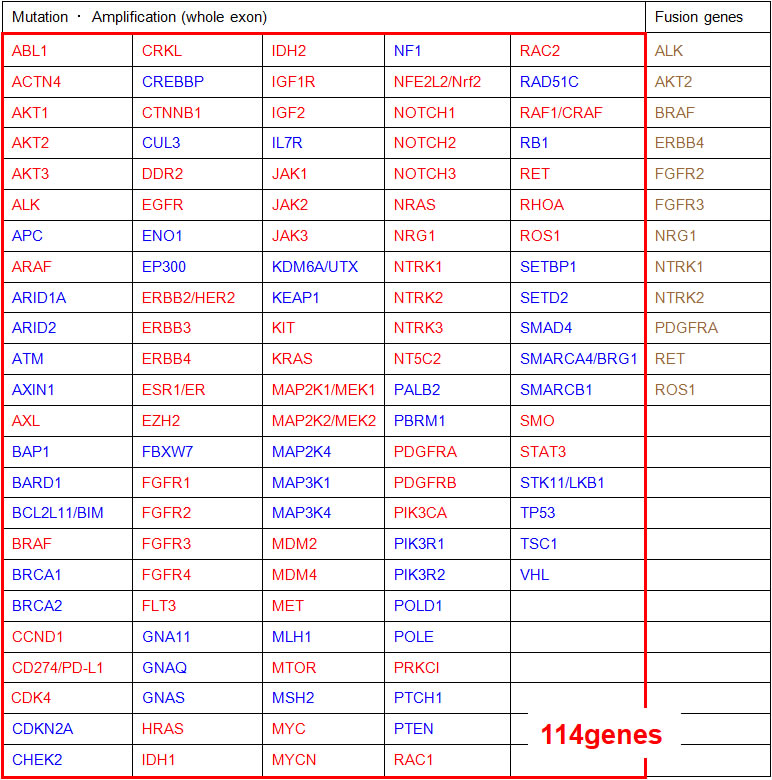
Mutations tested on NCC oncopanel
114 gene mutations are tested on "NCC oncopanel". They can be divided into gain-of function genes and loss-of-function genes. In the table below, 3 types of genes are highlighted: the genes (in red) can assist treatment decision when gain of function is detected, the genes (in blue) can assist treatment decision when loss of function is detected, and finally fusion genes (in brown, for 12 of the 114 genes) are investigated for improved treatment decision
Fees / expenses
All expenses required for genetic analysis, which is advanced medical care, will be borne by patients. Other examination and consultation expenses will be charged in line with other healthcare offered under general insurance coverage.
The following fees will be charged even if no genetic aberrations or actionable mutations are found.
Genetic sequencing test fee
Approximately JPY 670,000 (Partially funded by research, patient's expense at about JPY 470,000)
In addition, the estimated costs* for the examination itself, and consultations necessary for this study will be approximately JPY 25,000 (when 30% is borne by patient)
*variable depending on the use of biopsy. The cost of treatment following the results is not included.
Duration of the research (plan)
Registration period: 1 year (9-April-2018 to 31-Mar-2019)
Follow up: half a year (1-Apr-2019 to 30-Sep-2019)
Registration of subjects
205 to 350 subjects
Inquiries from patients etc
National Cancer Center Hospital Consultation, Counseling and Support Service Center
Telephone:03-3547-5293 (weekdays 9:00 ~ 12:00,13:00 ~ 16:00)
Inquiries from press
National Cancer Center – Planning Strategy Bureau – Public Relations Planning Office
5-1-1 Tsukuji, Chuo-Ku, Tokyo, 104-0045 Japan
Telephone: 03-3542-2511 FAX:03-3542-2545 E-mail: ncc-admin●ncc.go.jp(●replace to @)
