Home > Information > press release > Test Result of New Antiemetic Therapy for Chemotherapy-induced Nausea and Vomiting Proves Higher Efficacy Than Standard Antiemetic Therapy
Test Result of New Antiemetic Therapy for Chemotherapy-induced Nausea and Vomiting Proves Higher Efficacy Than Standard Antiemetic Therapy
December 11, 2019
Shizuoka Cancer Center
National Cancer Center Japan
Japan Supportive, Palliative and Psychosocial Oncology Group
Japan Agency for Medical Research and Development
The research group (Representative: Masakazu Abe, M.D., Ph.D., Division of Gynecology, Shizuoka Cancer Center, Secretariat: Hironobu Hashimoto, Department of Pharmacy, National Cancer Center Hospital) involving 30 medical institutions nationwide led by the Shizuoka Cancer Center (SCC, President: Ken Yamaguchi, M.D., Ph.D., Director: Mitsuru Takahashi, M.D., Ph.D., located in Nagaizumi-cho, Sunto-gun, Shizuoka Pref.), the National Cancer Center Japan (NCC, President: Hitoshi Nakagama, M.D., D.M.Sc., located in Chuo-ku, Tokyo) and the National Cancer Center Hospital (NCCH, Director: Toshirou Nishida, M.D., Ph.D.) announced the efficacy of the new antiemetic therapy for chemotherapy-induced nausea and vomiting with the test results of the phase III randomized-controlled trial “J-FORCE TEST (J-SUPPORT 1604) “ conducted under the initiatives of doctors and pharmacists.
Nausea and vomiting are two major adverse events of anti-cancer drug treatment, and antiemetic therapy is a supportive care*1 for alleviating the agonizing symptoms which cancer patients receiving chemotherapy often experience. This newly-developed antiemetic therapy involves olanzapine (an antipsychotic drug), which has been proven as effective for controlling chemotherapy-induced nausea and vomiting but hasn’t been widely used due to the side effects including somnolence and lightheadedness. The research group achieved success in preventing these side effects by decreasing the dose and adjusting the dosage timing, and in obtaining higher continuous antiemetic effects than the conventional standard antiemetic therapy.
More precisely, for the complete vomit suppression rate, which is the most critical index for a study of nausea and vomiting, they achieved an improvement by 13% in the delayed phase (i.e. the period from day 2 to day 5 after the start of chemotherapy) for which the improvement had been the most highly needed.
It is expected that this new antiemetic therapy will be internationally recognized to be adopted as a new standard antiemetic therapy in the near future, as this study result has proven effectiveness of it for preventing nausea and vomiting, as well as for controlling olanzapine-induced somnolence and lightheadedness in the morning after.
At the 2019 annual meeting of ASCO (the American Society of Clinical Oncology), this study was awarded the “Best of ASCO,” and was also awarded the “Best of ESMO” at the 2019 ESMO (the European Society for Medical Oncology) annual meeting. Furthermore, it was also recognized in the Japanese domestic societies and received the “Excellent Presentation” awards at the annual meetings of the Japanese Association of Supportive Care in Cancer, the Japan Society of Clinical Oncology and the Japanese Society of Clinical Oncology. The detailed content of this study was published in “The Lancet Oncology,” a highly-acclaimed international medical journal, on December 11, 2019.
This study has been supported by research funding of the Japan Supportive, Palliative and Psychosocial Oncology Group (J-SUPPORT), who has been committed to establish a permanent framework for multi-institutional collaborative clinical tests and clinical studies, and of the Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT) certified as “a research on development of cancer supportive care based on scientific evidences” by the Japan Agency for Medical Research and Development (AMED).
Summary of the J-FORCE clinical test
Background
Among various chemotherapy-induced adverse events, nausea and vomiting are experienced by more than half of cancer patients receiving chemotherapy, and they may possibly prevent the patients from continuing or completing their treatments, which will inevitably impact on their prognoses. For instance, cisplatin, which is being used as a standard treatment for cancers in lung, esophagus and womb, has an emetogenic risk as high as 90%, and a patient will feel just like carsick for several days after starting taking it. Therefore, continuous control of nausea and vomiting is required in order to retain the quality of the patient’s life. However, with the current standard antiemetic therapy, about 90% of nausea and vomiting can be controlled but it will only be for the acute phase, 24 hours after the first dose. The efficacy rate after the 24 hours will go down as low as 65% in the delayed phase (from day 2 to day 5 after the first dose), which proves the high need of continuous antiemetic effect.
In this study, a drug called olanzapine (an atypical antipsychotic drug) featured by very limited adverse events while conventional antipsychotic drugs are typically associated with many of them, has been used. Various studies already proved antiemetic effects of olanzapine for chemotherapy-induced nausea and vomiting in cancer patients in the US and some other countries, and the efficacy was well-confirmed. However, most of them involved high dose (10 mg) of olanzapine and induced strong somnolence and lightheadedness, which didn’t provide enough evidence for its safety before being widely used in Europe and Japan.
Therefore, two pilot studies were initiated in order to assess the antiemetic effect of olanzapine with lower dose, 5 mg, in Japan. One was the phase II study conducted by the SCC, which was to compare antiemetic effects of olanzapine 5 mg in combination with the standard antiemetic therapy, and the standard antiemetic therapy as it is. The result proved a higher antiemetic effect of the former. The other study was the randomized-and-controlled phase II study conducted by the Department of Pharmacy at the NCCH, which was to evaluate the efficacy of olanzapine 5 mg compared with 10 mg, the European standard dose. The result proved that olanzapine 5 mg would provide the same-leveled antiemetic effect as 10 mg, but would induce less somnolence. Although these were both the phase II studies, they were the first trials in the world evaluating the efficacy of olanzapine 5 mg in combination with the standard antiemetic therapy for cisplatin-based chemotherapy. Based on these pilot study results, this study was planned and designed.
Features
There are two major features about this study: It involves olanzapine 5 mg, and the dosage timing is after dinner in the evening instead of before going to bed. It is because it takes 3 or 4 hours after taking olanzapine for the blood level to become the highest. This means that the patient will be asleep when somnolence, an olanzapine-induced side effect, becomes the strongest. This study design controlling somnolence and lightheadedness in the morning after has been very highly acclaimed internationally.
Methods
As this study was designed to compare the standard triple antiemetic therapy (involving serotonin receptor antagonist*2, neurokinin 1 receptor antagonist*3 and steroid) and the combination therapy with olanzapine 5 mg added to it, the patients were randomly divided into two groups, and provided with the therapies including placebo. Neither the patients themselves nor the nurses and doctors knew which group they belonged to. This is called a placebo-controlled double-blind phase III study and is supposed to be the best and the most scientific method when two therapies are compared to prove which one is better and more effective (refer to chart 1).
Chart 1. Placebo-controlled double-blind phase III study
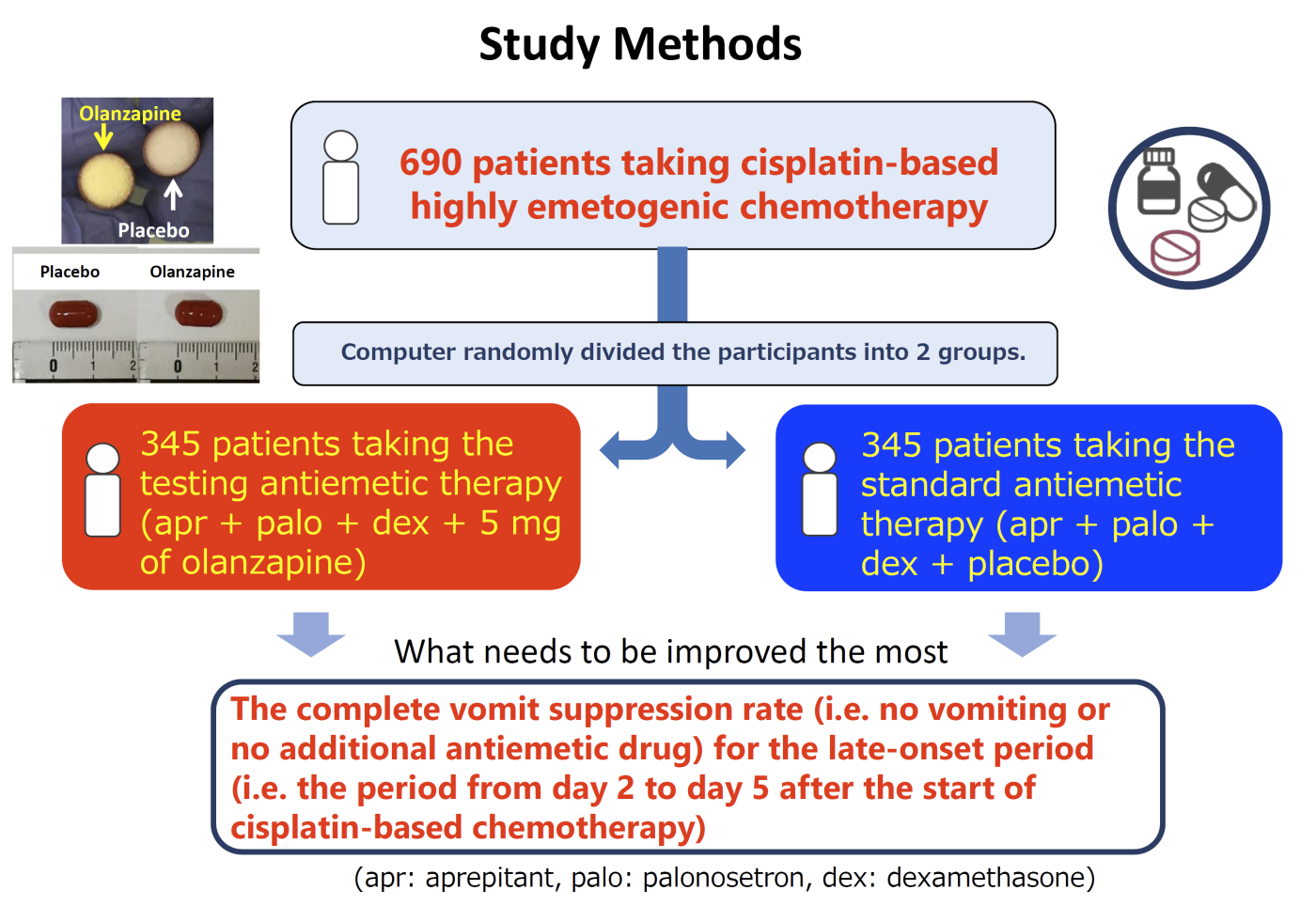
Each group, one involved olanzapine and the other involved placebo, was evaluated for the 3 indexes as shown in the table below for the acute phase, delayed phase and entire period (i.e. period covering acute and delayed phases both). What needed to be improved the most was the complete vomit suppression rate (complete response, CR) for the delayed phase, in which the study group had been keen to see the improvement.
Table 1. Evaluating indexes used in the antiemetic therapy tests
|
evaluating index |
vomiting |
nausea |
|
|
CR (complete response) Complete suppression of vomiting |
none |
none |
no object |
|
CC (complete control) Complete suppression of nausea and vomiting |
none |
none |
No significant (none or mild) nausea |
|
TC (total control) Total control of nausea and vomiting |
none |
none |
none |
(rescue therapy: On-demand dose of antiemetic drug provided when nausea is intolerable.)
In addition to evaluating the antiemetic effects, each patient participated in the study was asked to keep records in their symptom reports to tell if they had somnolence, which is a major adverse event of olanzapine, during the day, if they slept well during the night, and if their appetites were lowered or not (because olanzapine has an orexigenic effect).
In this study, 710 patients with lung, esophagus and womb cancers starting cisplatin-based chemotherapy for the first time in 30 medical institutions nationwide participated and cooperated.
Results
- The complete vomit suppression rate for the delayed phase (CR), which was the main evaluating index, was significantly better in the olanzapine group with 79% than the placebo group with 66%. The improvement by 13% filled the criterion “improvement by 10% or higher” of the international standard for efficacy of a newly-developed therapy (refer to chart 2).
- Except the total nausea-and-vomiting control rate (TC) for the acute phase, in all the sub-evaluating indexes, the results were significantly better in the olanzapine group. This means that improvements were proven for the rate of staying without either vomiting or rescue therapy as well as for the severity of nausea (refer to chart 2).
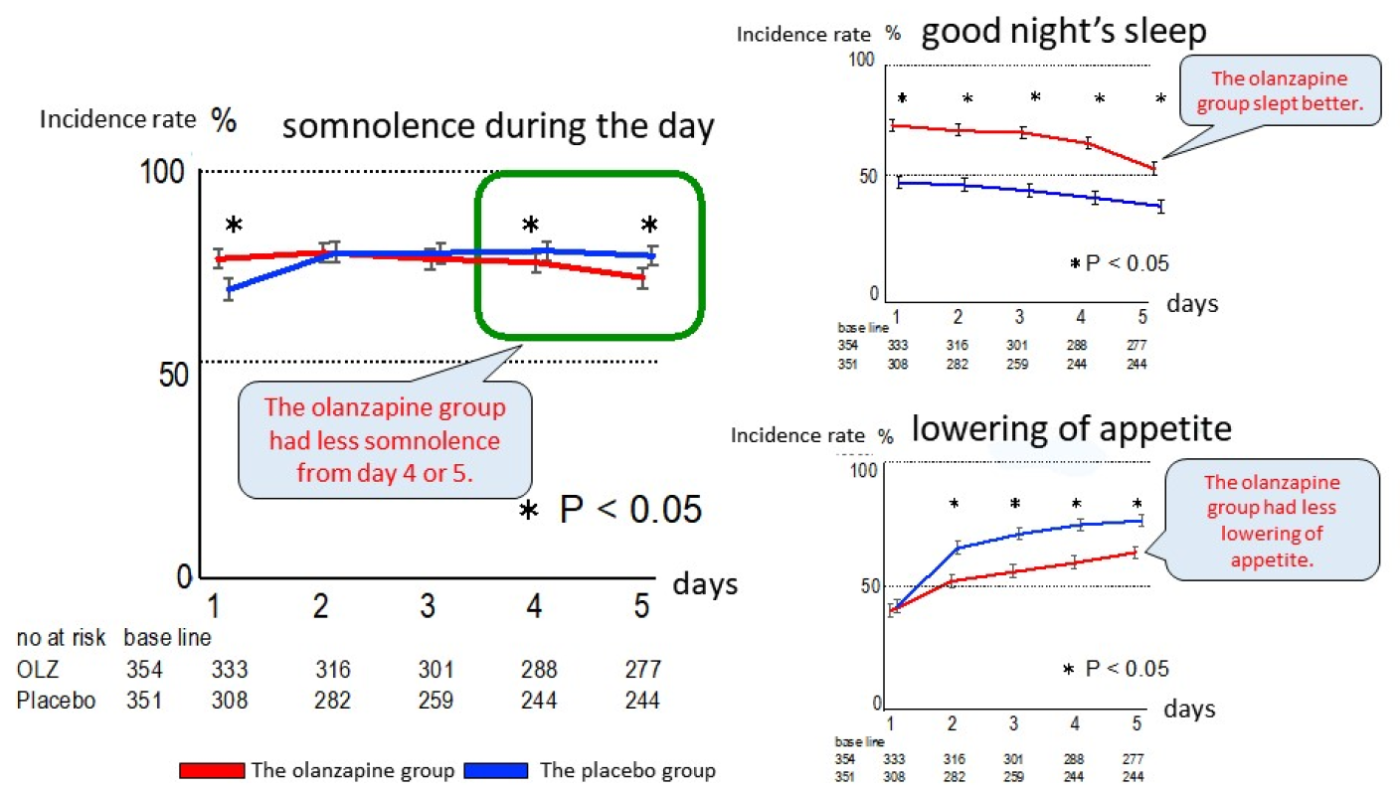
- There was not much difference identified in somnolence during the day between the olanzapine group and the placebo group, and even for “no asomnia = good night’s sleep,” the result was better in the olanzapine group. It was suggested that moving the dosage timing of olanzapine from before going to bed, which is conventionally being done, up to after dinner in the evening, impacted on the sleeping pattern with somnolence, the side effect of olanzapine, appearing just in time for falling asleep at night and not during the day. For “lowering of appetite,” the results for the olanzapine group were significantly low, which suggested the efficacy in preventing lowering of appetite. These results were not included in the past study reports involving olanzapine 10 mg conducted in the US (refer to chart 3).
Chart 2. Evaluation results of the efficacy
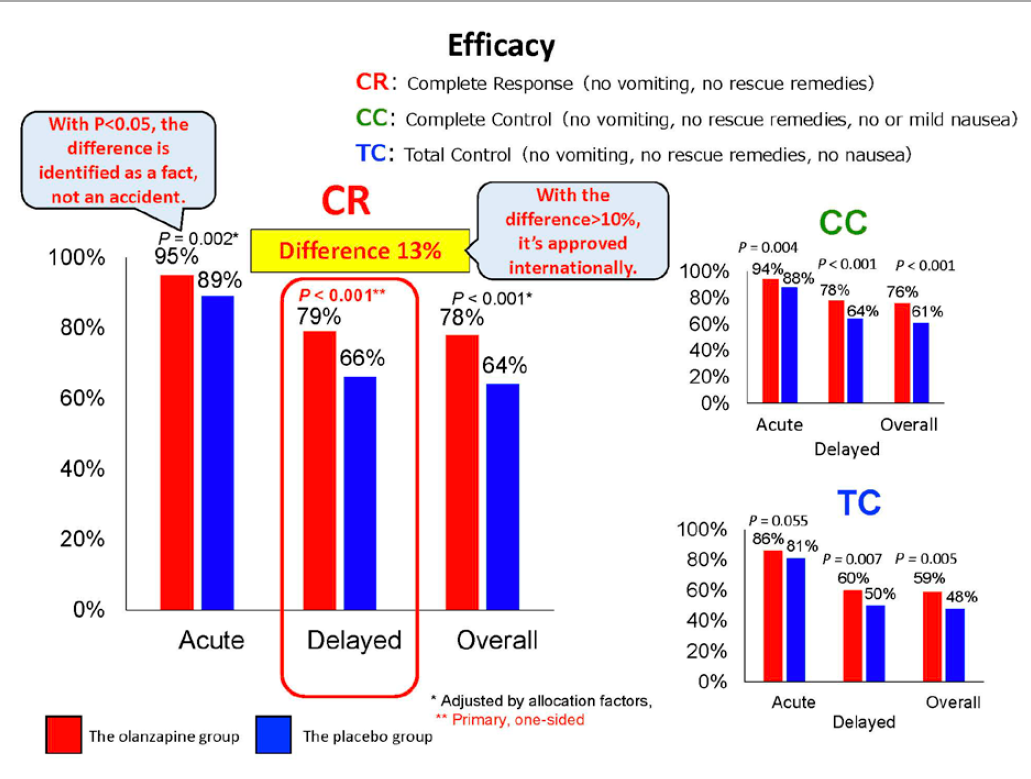
Chart 3. Incidence rates for “somnolence during the day,” “no asomnia” and “lowering of appetite”
Results
This study proved the antiemetic effect of olanzapine while preventing somnolence and lightheadedness during the day, and at the same time, suppressing nausea and vomiting in the delayed phase, when the improvement for the continuous antiemetic effect had been needed very badly. It is expected that this new therapy with olanzapine 5 mg in combination with the standard antiemetic therapy will be adopted in the international guidelines as a new standardized antiemetic therapy for cisplatin-based chemotherapy.
Journal publication
-
Name of the journal: The Lancet Oncology
-
Article title: Olanzapine 5 mg in combination with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based chemotherapy (J-FORCE): a randomized, double-blind, placebo-controlled, phrase 3 trial
-
Authors: Hironobu Hashimoto (First author), Masakazu Abe (Corresponding author)
Research support organization
The Japan Supportive, Palliative, and Psychological Oncology Group (J-SUPPORT)
(http://www.j-support.org/ linked at external site.)
J-SUPPORT is a research organization with the development strategy in supportive care aiming at supporting researches in the field from developing to prevalence and implementation in the all-Japan system. It was established in February, 2016, with the funds for R & D of the NCC (27-A-3). The secretariat has been located at the Innovation Center for Supportive, Palliative and Psychosocial Care and the Center for Public Health Sciences in the NCCH since then.
They have had 60 research projects offered from all over Japan, and 12 of them have been approved as quality projects responding to unmet needs. This study is the first clinical trial among them with the evaluation results obtained from patients’ participation.
Research funding agency
The Japan Agency for Medical Research and Development (AMED)
Practical Research for Innovative Cancer Control
- Name of the research development project: A randomized, double-blind, placebo-controlled phase III study evaluating olanzapine combined with standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based, highly emetogenic chemotherapy
- Representative: Masakazu Abe, M.D., Ph.D.
- Secretariat: Hironobu Hashimoto, the Department of Pharmacy, NCCH
The study group
30 medical institutions: 10 cancer hospitals, 12 university hospitals and 8 general hospitals
(Shizuoka Cancer Center, National Cancer Center Hospital, Hokkaido Cancer Center, Sapporo Medical University, Hakodate Central General Hospital, Hakodate Municipal Hospital, Niigata Cancer Center Hospital, Gunma Prefectural Cancer Center, Saitama Cancer Center, National Cancer Center Hospital East, Cancer Institute Hospital of JFCR, Tokyo Medical University Hospital, Showa University Hospital, Hamamatsu University Hospital, Toyama Kouseiren Takaoka Hospital, Gifu University Hospital, Osaka City General Hospital, Osaka University Hospital, Wakayama Medical University Hospital, Kansai Rousai Hospital, Hyogo Prefectural Amagasaki General Medical Center, Kobe Minimally Invasive Cancer Center, Tottori University Hospital, Hiroshima City Hiroshima Citizens Hospital, Kochi Health Sciences Center, Shikoku Cancer Center, Fukuoka University Hospital, Kurume University Hospital, Oita University Hospital, Kyushu University Hospital)
Glossary
*1. Supportive care
Supportive care in cancer is the prevention, treatment and management of the adverse events, complications and after effects of cancer and its treatment. This includes management of physical and psychological symptoms and side effects across the continuum of the cancer experience from diagnosis through treatment to post-treatment care including rehabilitation and end-of-life care. Enhancing oral care and rehabilitation, secondary cancer prevention, survivorship, and end-of-life care are integral to supportive care.
Following febrile neutropenia (FN), one of chemotherapy-induced adverse events with high needs for managements, nausea and vomiting seem to be almost entirely brought under control with this new antiemetic therapy. However, there are still many other chemotherapy-induced adverse events, including numbness or fatigue, that are still in need of effective control therapies. Although the evaluation method for those adverse events highly relies on self-reported symptoms by the patients, the process for establishing symptom-control therapies has just started with a definition of each symptom.
*2. Serotonin receptor antagonist
Drug with antiemetic effect, inhibiting serotonin, being released from intestinal mucous membrane, from linking with nerve stimulations at serotonin receptors.
*3. Neurokinin 1 receptor antagonist
Drug with antiemetic effect, inhibiting neurokinin receptors in central nervous system from linking with substance P, one of neural transmitter substances.
For more information, please contact below.
Public Relations, Management Center, Shizuoka Cancer Center
1007 Shimonagakubo, Nagaizumi-cho, Sunto-gun, Shizuoka Pref. 411-8777
Tel: +81-55-989-5222
Fax: +81-55-989-5793
Email: info●scchr.jp(●を@に置き換えてください)
Public Relations, Strategic Planning Bureau, National Cancer Center Japan
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045
Tel.: 03-3542-2511
Fax: 03-3542-2545
Email: ncc-admin●scc.go.jp(●を@に置き換えてください)
Inquiry about AMED
Division of Cancer Research, Department of Research Promotion
Japan Agency for Medical Research and Development (AMED)
Yomiuri Shimbun Bldg., 1-7-1 Otemachi, Chiyoda-ku, Tokyo 100-0004
Tel.: 03-6870-2221
Email: cancer●amed.go.jp(●を@に置き換えてください)
