Home > Information > press release > A novel biomarker for predicting clinical efficacy of PD-1 blockade therapies
A novel biomarker for predicting clinical efficacy of PD-1 blockade therapies
Highlights
A research team mainly based at National Cancer Center identified a novel biomarker which can predict the clinical efficacy of PD-1 blockade therapies. Precision medicine utilizing the biomarker predicting clinical efficacy of immunotherapy is anticipated.
The team developed an evaluation system to measure the biomarker from phenotypes of tumor-infiltrating lymphocytes, a technical challenge, in collaboration with Nippon Becton Dickinson Company. The breakthrough will promote the development of diagnostic kits for use in clinical settings.
Clinical trials to validate the developed biomarker will follow towards realizing precision medicine in cancer immunotherapy.
Summary
We have identified a novel biomarker that predicts the therapeutic effects of PD-1 blockade therapies and have also developed a new measuring tool for evaluating the biomarker. Clinical trials will follow to realize the clinical application of this biomarker.
Immunotherapies with immune checkpoint inhibitors have been deployed for the treatment of various types of cancer. However, there are many issues; patients showing therapeutic effects are limited to only 20-30%, serious adverse effects are observed in some patients, and it is expensive.
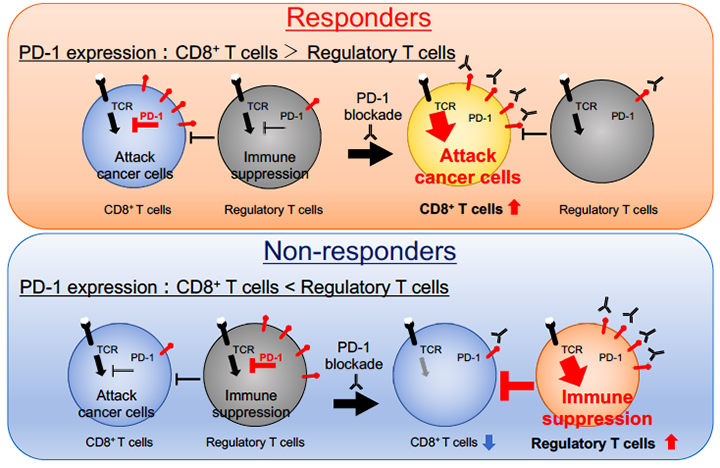
In this study, we utilized pre-treatment tissue samples from patients with malignant melanoma, lung cancer and gastric cancer treated with PD-1/PD-L1 blockade (nivolumab, pembrolizumab or atezolizumab) to evaluate immunological phenotypes of tumor-infiltrating lymphocytes (TILs). As a result, PD-1 expression balance between tumor-infiltrating effector and regulatory T cells correlated with antitumor effects of the treatment, and predicted the clinical efficacy with high accuracy.
For identifying the biomarker, the research team collaborated with Ono Pharmaceutical. For clinical application of this biomarker, Nippon Becton Dickinson Company has jointly developed a new measuring tool for evaluating PD-1 expression balance in TILs, which was considered technically difficult. National Cancer Center continues to evaluate the biomarker in clinical settings.
The results of the research were published in the electronic version of the American scientific journal Nature Immunology on August 31, 2020.
This research was led by Dr. Hiroyoshi Nishikawa, Chief of Division of Cancer Immunology, Research Institute/Exploratory Oncology Research & Clinical Trial Center (EPOC), National Cancer Center (Professor of Department of Immunology, Nagoya University Graduate School of Medicine), Dr. Hiroyuki Mano, Chief of Division of Cellular Signaling, National Cancer Center Research Institute, and Dr. Toshihiko Doi, Deputy Director of National Cancer Center Hospital East, Dr. Shigeyuki Matsui, Professor of Department of Biostatics, Nagoya University Graduate School of Medicine, and Hitachi, Ltd.
Research Paper
Journal name
Nature Immunology
Title
The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies
Authors
Shogo Kumagai1,2,11, Yosuke Togashi1,11, Takahiro Kamada1,2,11, Eri Sugiyama1,2, Hitomi Nishinakamura1, Yoshiko Takeuchi1, Kochin Vitaly2, Kota Itahashi1,Yuka Maeda1, Shigeyuki Matsui3, Takuma Shibahara4, Yasuho Yamashita4, Takuma Irie1, Ayaka Tsuge1,2, Shota Fukuoka1, Akihito Kawazoe5, Hibiki Udagawa6, Keisuke Kirita6, Keiju Aokage7, Genichiro Ishii8, Takeshi Kuwata8, Kenta Nakama9, Masahito Kawazu10, Toshihide Ueno10, Naoya Yamazaki9, Koichi Goto6, Masahiro Tsuboi7, Hiroyuki Mano10, Toshihiko Doi5, Kohei Shitara5 and Hiroyoshi Nishikawa1,2*
Affiliations: 1Division of Cancer Immunology, Research Institute/Exploratory Oncology Research & Clinical Trial Center (EPOC), National Cancer Center, Tokyo 104-0045/Chiba 277-8577, Japan, 2Department of Immunology, 3Department of Biostatics, Nagoya University Graduate School of Medicine, Nagoya 466–8550, Japan, 4Research and Development Group Hitachi Limited, Tokyo 185-8601, Japan. 5Department of Gastroenterology and Gastrointestinal Oncology, 6Department of Thoracic Oncology, 7Department of Thoracic Surgery and 8Pathology and Clinical Laboratories, National Cancer Center Hospital East, Chiba 277-8577, Japan, 9Department of Dermatologic Oncology, National Cancer Center Hospital, Tokyo 104-0045, Japan, 10Division of Cellular Signaling, National Cancer Center Research Institute, Tokyo 104-0045, Japan.11These authors contributed equally to this study.
DOI
10.1038/s41590-020-0769-3
Data of publication
August 31, 2020
URL
https://www.nature.com/articles/s41590-020-0769-3(I inked at external site)