Home > Information > press release > NATIONAL CANCER CENTER AND EISAI COMMENCE JOINT RESEARCH AND DEVELOPMENT PROJECT “BASIC RESEARCH ON THE DRUG DISCOVERY AND DEVELOPMENT TO ACCELERATE DEVELOPMENT OF ANTICANCER DRUGS IN TREATMENT OF PATIENTS WITH RARE CANCERS AND REFRACTORY CANCERS”, USING PDX WITH HIGH PREDICTABILITY OF CLINICAL OUTCOMES, AND CANCER GENOME DATA
NATIONAL CANCER CENTER AND EISAI COMMENCE JOINT RESEARCH AND DEVELOPMENT PROJECT “BASIC RESEARCH ON THE DRUG DISCOVERY AND DEVELOPMENT TO ACCELERATE DEVELOPMENT OF ANTICANCER DRUGS IN TREATMENT OF PATIENTS WITH RARE CANCERS AND REFRACTORY CANCERS”, USING PDX WITH HIGH PREDICTABILITY OF CLINICAL OUTCOMES, AND CANCER GENOME DATA
May 14, 2021
National Cancer Center Japan
Eisai Co., Ltd.
Highlights
- The National Cancer Center Japan and Eisai will work to create therapeutic drugs for rare cancers and refractory cancers with high unmet medical needs through this R&D project.
- Through this R&D project, both parties will establish a unique system for developing novel anticancer drugs to be carried out consistently from non-clinical research to clinical research, aiming to accelerate the development of Eisai’s anticancer drug candidates and get regulatory approval, by utilizing the Patient-Derived Xenograpft (PDX) library of animal models in which tumor tissue derived from Japanese cancer patients is transplanted into immunodeficient mice and the cancer genome data, that are possessed by the National Cancer Center Japan.
- This R&D project is to be carried out with funding under the program “Cyclic Innovation for Clinical Empowerment (CiCLE)” established by the Japan Agency for Medical Research and Development (AMED).
Summary
The National Cancer Center Japan (Location: Tokyo, President: Hitoshi Nakagama, “National Cancer Center”) and Eisai Co., Ltd. (Headquarters: Tokyo, CEO: Haruo Naito, “Eisai”) announced today that both parties have entered into a joint research and development (R&D) agreement concerning “Basic research on the drug discovery and development to accelerate development of anticancer drugs in treatment of patients with rare cancers and refractory cancers”, and that research activities have commenced. This R&D project is to be carried out with funding under the program “Cyclic Innovation for Clinical Empowerment (CiCLE)” established by the Japan Agency for Medical Research and Development (AMED).
Rare cancer is a disease for which it is difficult for pharmaceutical companies to develop new drugs solo due to the extremely small number of patients, and there are only a limited number of drugs for rare cancers that can be approved for manufacturing and marketing. Also, among certain types of cancer with a large number of patients, refractory cancers, for which standard treatment has not been established, face barriers to creating new drugs due to difficulty of research and development. In order to promptly deliver effective treatments to patients with rare or refractory cancers, it is essential to provide predictability of clinical outcomes with high accuracy and efficiency in non-clinical research that confirms in advance the effectiveness of the drug, to transfer non-clinical research seamlessly to clinical studies, and moreover to elucidate the mechanisms of drug resistance, actual therapeutic effects and side effects. Both parties aim to realize these capabilities in this R&D project using Patient-Derived Xenografts (PDX) library, a model in which cancer tissue derived from patients is transplanted into immunodeficient mice with high predictability of clinical outcomes, as well as cancer genome data.
The National Cancer Center is a leading institution in basic research, epidemiological research, and clinical studies for all cancer types including rare cancers in Japan. As of May 2020, with a grant from AMED CiCLE, the National Cancer Center has established a large-scale PDX library,"J-PDX", derived from Japanese cancer patients with information on clinical outcomes, and has also completed the development of research infrastructure and framework. More than 410 types of PDX, including rare cancers and refractory cancers, have already been established in J-PDX (as of March 2021).
Eisai is advancing the research and development of new anticancer drugs, targeting cancer genomics and the tumor microenvironment, with its experience and knowledge from globally approved in-house discovered compounds: microtubule dynamics inhibitor eribulin mesylate (product name: Halaven®) and multiple receptor tyrosine kinase inhibitor lenvatinib mesylate (product names: Lenvima®).
In research and development under the agreement, the National Cancer Center and Eisai will jointly conduct tumor-agnostic non-clinical research on new drug candidates created by Eisai, using J-PDX with relevant clinical and biological information, and will determine the drugs and the target cancer types to be transferred to clinical studies. After that, investigator-initiated studies will be conducted for rare cancers and refractory cancers in order to confirm clinical benefits of these new drugs, with the aim to apply for approval of them. Further, both parties will consider expanding into new drug discovery research, with establishment of PDX with tumor tissues taken from patients before and after treatment, comparative analyses of drug responsiveness and cancer genome, as well as search for new drug discovery targets and elucidation of drug resistance mechanisms. Through these efforts, both parties aim to establish a drug discovery and development research system that accelerates the development of new anticancer drugs in Japan.
Through research and development based on the agreement, the National Cancer Center and Eisai will work to develop therapeutic drugs for rare cancers and refractory cancers with high unmet medical needs, thereby aiming to make continuous efforts to meet the diversified needs of, and increase the benefits provided to patients with cancer, their families, and healthcare professionals.
Glossary
About rare cancer
Rare cancer is defined, by the Ministry of Health, Labour and Welfare, Japan, as a carcinoma that meets the following two conditions; (1) The incidence (morbidity) of the cancer per 100,000 people is less than 6 cases per year, and (2) Due to the small number of affected people, there are more challenges in medical care and treatment for the cancer than in other cancer types. The number of patients with rare cancer is small, and there are few doctors and medical institutions that specialize in the cancer type. Therefore, it is difficult to establish clinical practice guidelines for rare cancers, and to develop effective diagnostic and therapeutic methods for rare cancers, and so put it to practical use. In addition, the lack of clinical evidence and information from medical institutions poses a challenge for rare cancers.
About J-PDX
One of the challenges in the development of anticancer drugs so far is that the predictive ability of the experimental model using the cell line used to predict the therapeutic effect is low (Fig. 1).
On the other hand, PDX is an animal model in which cancer tissue of a cancer patient is transplanted into an immunodeficient mouse to reproduce a tumor, and has a high recall rate because it can retain the characteristics of cancer tissue. So, PDX has been reported to have a high recall rate. Utilization of PDX for drug discovery is rapidly advancing (Fig. 2).
The large-scale PDX library "J-PDX" derived from Japanese cancer patients established by the National Cancer Center has the following features, and its use is expected to activate the development of new anticancer drugs and accelerate its development; 1. Having established cross-organ PDX and focused on rare cancers (osteosarcoma, rhabdomyosarcoma, etc.) and cancers that are common in Asia in addition to major cancers, 2. Having established PDX not only from surgical specimens but also from drug-resistant specimens, 3. PDX with detailed clinical information including treatment history.
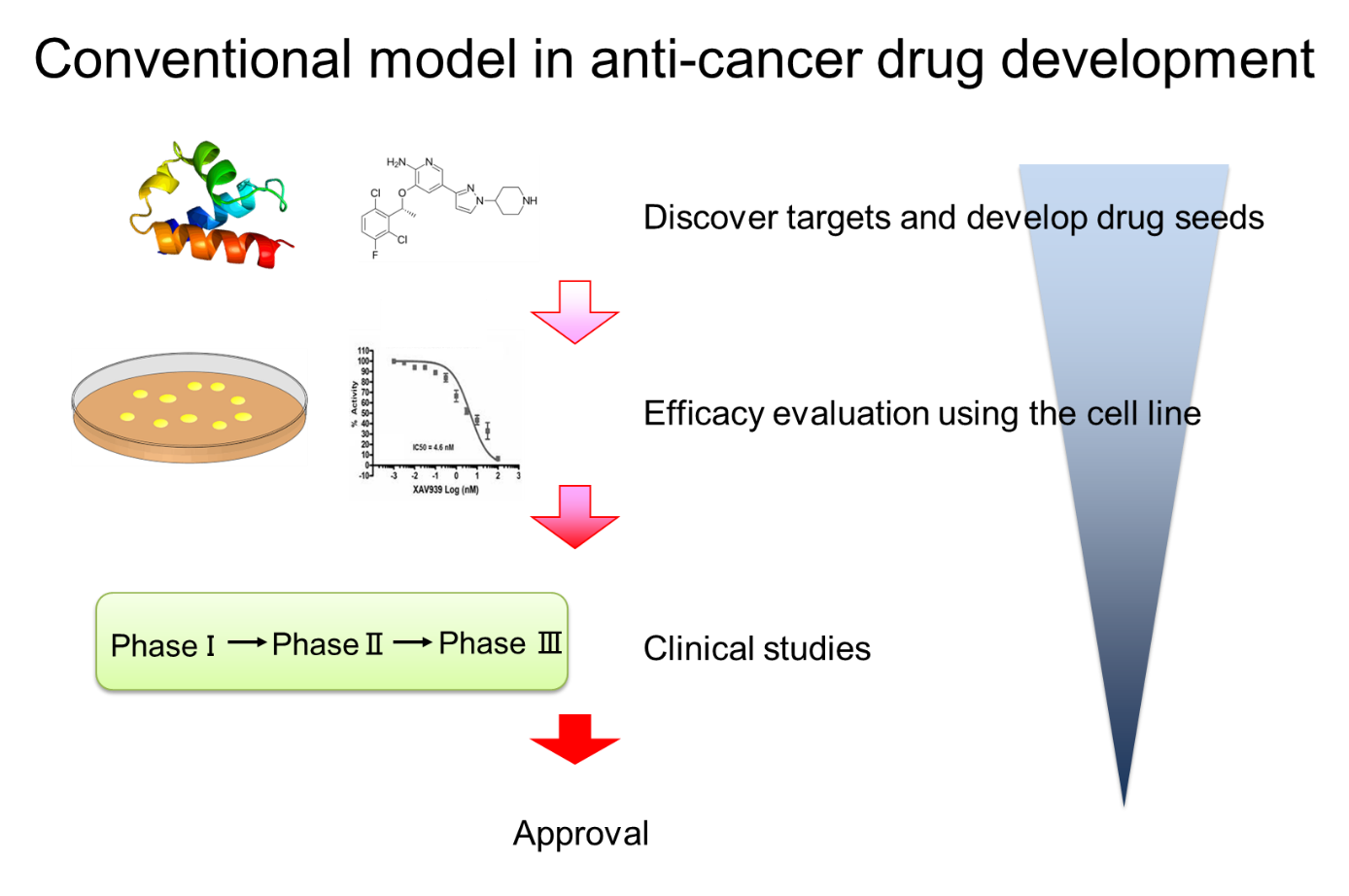
Fig.1
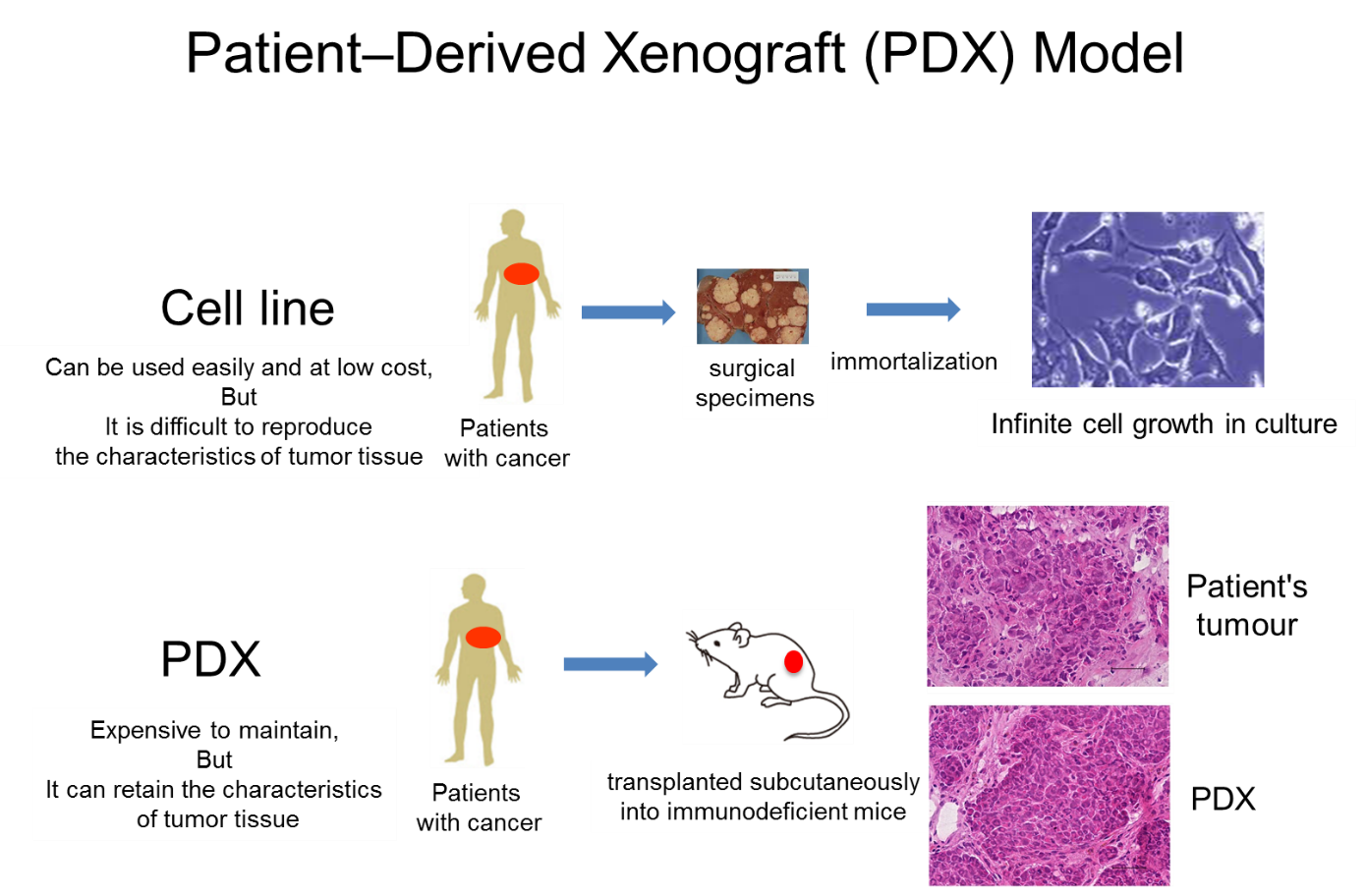
Fig.2
About CiCLE
AMED’s CiCLE is a grant program to promote the establishment of infrastructure (including human resources) to respond to medical needs and the creation of an environment for open innovation and venture development based on industry-academia-government collaboration
About National Cancer Center Japan
The National Cancer Center was founded in 1962 as a national institution to be a hub for cancer treatment and research. Since its establishment, as an institution that serves as a nucleus of clinical research and development for cancer treatment, the Center has led cutting-edge research aimed at elucidating the pathophysiology of cancer and developing treatments based on it, with integrating its insight and experience accumulated by experts at the National Cancer Center Research Institute, the National Cancer Center Hospital (Tsukiji campus) and the National Cancer Center Hospital East (Kashiwa campus). In addition, the number of clinical trials conducted is one of the highest in Japan, and also both the Hospital and the East Hospital have been accredited as a “clinical research core hospital” under the Medical Care Act, since then leading world class clinical research such as Phase I trials and investigator-initiated trials, contributing to the establishment of new standard treatments.
In particular, at present, the Center is vigorously reinforcing its research and clinical systems to address unmet medical needs, developing a framework based on genomic information to provide personalized treatment as well as systemized preemptive medical care for individual patients, and policy advocacy. The Center is actively collaborating with companies, industrial and academic research institutions and universities.
About Eisai Co., Ltd.
Eisai Co., Ltd. defines our corporate mission as "giving first thought to patients and their families and to increasing the benefits health care provides," which we call our human health care (hhc) philosophy. With approximately 10,000 employees working across our global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products to address unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology. As a global pharmaceutical company, our mission extends to patients around the world through working with key stakeholders to improve access to medicines in developing and emerging countries.
For further information on Eisai Co., Ltd., please visit https://www.eisai.com(linked at external site)
Media Inquiries
National Cancer Center Japan Strategic Planning Bureau Office of Public Relations
5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
E-mail:ncc-admin●ncc.go.jp(●を@に置き換えてください)
Eisai Co., Ltd. Public Relations Department
TEL : +81-(0)3-3817-5120
