Home > Information > press release > Teijin, J-TEC, Mitsui Fudosan and NCC to Establish Regenerative Medicine Platform
Teijin, J-TEC, Mitsui Fudosan and NCC to Establish Regenerative Medicine Platform
September 27, 2022
In Japanese
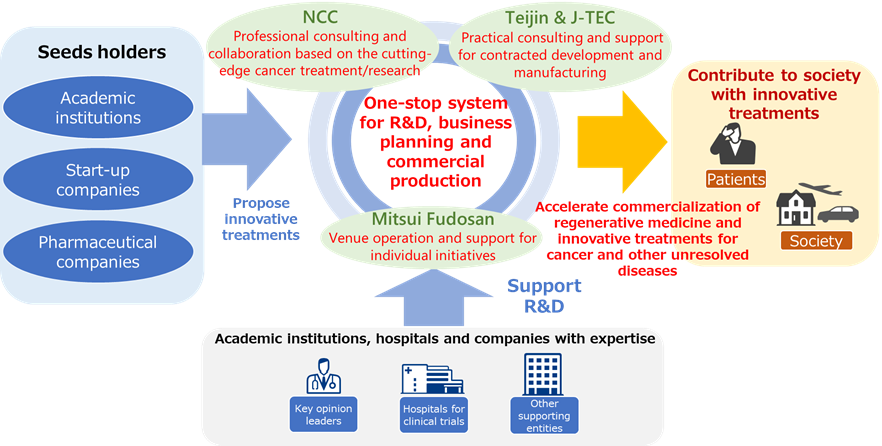
Tokyo, Japan, September 27, 2022 --- Teijin Limited, Japan Tissue Engineering Co., Ltd. (J-TEC), Mitsui Fudosan Co., Ltd. and the National Cancer Center (NCC) jointly announced today that they will establish a regenerative medicine platform in Kashiwanoha Smart City, a life science business park in Kashiwa-shi, Chiba Prefecture, Japan. The platform supports the development of innovative treatments for diseases with unmet needs such as cancer. It serves a one-stop system supporting research and development, business plan formulation, and commercial production of regenerative medicine products. In particular, the four partners aim to accelerate the commercialization of regenerative medicine and innovative treatments for cancer. As part of the plan, the partners already have begun to support seed holders.
Looking at the four partners: NCC provides cutting-edge cancer treatments and produces drug discovery seeds; J-TEC has extensive experience in product development, manufacturing and marketing as a pioneer of regenerative medicines; Teijin boasts expertise in drug research, development and marketing; and Mitsui Fudosan builds spaces and communities for innovation in life sciences. Their envisioned platform, taking advantage of the physical and functional proximity of the four parties, is expected to quickly and efficiently provide solutions for manufacturing and clinical development, business planning and commercial production. The contract development and manufacturing organization (CDMO) served by Teijin and J-TEC will be located in Mitsui Link-Lab Kashiwanoha 1 operated by Mitsui Fudosan, and is adjacent to the NCC Hospital East and NCC Exploratory Oncology Research & Clinical Trial Center.
The platform will also support projects of other organizations that have seeds. NCC will provide professional consulting and collaboration with seeds holders and investigator-initiated clinical trials. Teijin and J-TEC offer practical consulting and support for contracted development and manufacturing. Mitsui Fudosan will be responsible for venue operation and providing support for individual initiatives.
In preparation to start up the CDMO facility scheduled to open in January 2024, the platform will promote the development of regenerative medicines among academic institutions, start-up companies, pharmaceutical companies and others. It also will solicit research and development and clinical collaborations with medical institutions as well as key opinion leaders (KOL) and business enterprises in the fields of cancer and regenerative medicine.
With this agreement, the four parties will strive to stimulate demand and build up their platform, aiming eventually at establishing a structure capable of supporting about 10 projects annually with the purpose of bringing regenerative medicines to market. Teijin, in collaboration with J-TEC, will invest a substantial portion of its more than JPY 3 billion CDMO-related investment budget into the project, mainly for facilities and human resource training in Mitsui Link-Lab Kashiwanoha 1 over the next two to three years, thereby helping to establish a CDMO facility mainly for supporting the development of manufacturing methods as well as actual manufacturing. Ultimately, the four parties expect to contribute to global medical care by providing new options for patients waiting for innovative treatments.
According to the NCC, some one million people are diagnosed with cancer each year in Japan. Development of innovative treatments using regenerative medicines, including genetically modified immune cell therapy, also known as chimeric antigen receptor (CAR-T) cell therapy, has been ongoing worldwide in recent years. In addition, research and development of promising treatments and their seeds are under way. Meanwhile, the practical application of regenerative medicine requires specialized knowledge and practical experience regarding matters such as pharmaceutical approval and quality control. A wide range of commercialization knowhow also is required. As a result, many academic institutions and start-up companies are unable to commercialize their research and development independently. Teijin, J-TEC, Mitsui Fudosan and NCC, based on their clear understanding of such needs, have now agreed to establish a regenerative medicine platform to provide academic institutions with professional expertise and commercialization knowhow on a collaborative basis.

Kashiwa-no-ha Smart City, which is served by Kashiwa-no-ha Campus Station about 30 minutes by express train from central Tokyo, is an area with accumulation of Japan’s leading academic and medical institutions for some of Japan's leading academia and medical facilities. In addition to Mitsui Garden Hotel Kashiwa-no-ha Parkside, which supports cancer patients, the smart city has become a lively community of offices and commercial facilities. Mitsui Link Lab Kashiwanoha 1, located at 6-6-2 Kashiwanoha, Kashiwa-shi, Chiba Prefecture, comprises about 11,000 m2 of total floor space and offers rental space of about 8,000 m2.
About the Teijin Group
Teijin (TSE: 3401) is a technology-driven global group offering advanced solutions in the fields of environmental value; safety, security and disaster mitigation; and demographic change and increased health consciousness. Originally established as Japan's first rayon manufacturer in 1918, Teijin has evolved into a unique enterprise encompassing three core business domains: high-performance materials including aramid, carbon fibers and composites, and also resin and plastic processing, films, polyester fibers and products converting; healthcare including pharmaceuticals and home healthcare equipment for bone/joint, respiratory and cardiovascular/metabolic diseases, nursing care and pre-symptomatic healthcare; and IT including B2B solutions for medical, corporate and public systems as well as packaged software and B2C online services for digital entertainment. Deeply committed to its stakeholders, as expressed in the brand statement “Human Chemistry, Human Solutions,” Teijin aims to be a company that supports the society of the future. The group comprises some 170 companies and employs some 20,000 people across 20 countries worldwide. Teijin posted consolidated sales of JPY 926.1 billion (USD 7.2 billion) and total assets of JPY 1,207.6 billion (USD 9.4 billion) in the fiscal year that ended on March 31, 2022.
Please visit www.teijin.com(linked at external site)
About J-TEC
J-TEC (TSE: 7774) is a maker of regenerative medical products whose corporate vision is “creating a future for regenerative medicine,” and has been a member of the Teijin Group since March 2021. As Japan’s top runner in regenerative medicine, J-TEC obtained marketing approval for autologous cultured epidermis “JACE”, Japan’s first regenerative medical product, in October of 2007, and began marketing the product in January of 2009. J-TEC then went on to obtain marketing approval for Autologous Cultured Cartilage “JACC” in July of 2012, for Autologous Cultured Corneal Epithelium “Nepic” in March of 2020, and for Autologous Cultured Oral Mucosal Epithelium “Ocural” in June 2021. Of the 16 regenerative medical products that have been approved in Japan, four are J-TEC products. By making the most of the experience and knowhow cultivated through these achievements, J-TEC is engaging in contract development of regenerative medical products, consulting, and contract manufacture of specific cell-processed products.
Please visit www.jpte.co.jp/en/(linked at external site)
About Mitsui Fudosan Co., Ltd.
(Kashiwa-no-ha Smart City: https://www.kashiwanoha-smartcity.com/en/)(linked at external site)
Mitsui Fudosan is a comprehensive developer that creates new value by striving to resolve social issues through urban development. At Kashiwa-no-ha Smart City, Mitsui Fudosan aims to create a smart, compact city driven by data through the introduction of new technologies such as AI and IoT. It has been selected by the Ministry of Land, Infrastructure, Transport and Tourism as an advanced model project for a smart city towards realizing Society 5.0. Going forward, Mitsui Fudosan will work on developing smart medical institution services for health and medicine. In addition, the Mitsui Fudosan Group believes that it can contribute significantly to the realization of Society 5.0, which is advocated by the Japanese government, and to the achievement of the SDGs, by promoting ESG management, which means advancing businesses based on an awareness of the Environment (E), Society (S), and Governance (G).
About National Cancer Center Japan
The National Cancer Center Japan, established in 1962, is a leading medical institution in cancer treatment and research in Japan. It is actively involved in physician-led clinical trials and clinical research, and has produced a wealth of research results that have led to the development of new drugs.
Press Contact
Corporate Communications
Teijin Limited
+81 (0)3 3506 4055
pr●teijin.co.jp
Corporate Communication Office
Japan Tissue Engineering Co., Ltd.
+81 (0) 533 66 2020
jtec-info●jpte.co.jp
Public Relations Department
Mitsui Fudosan Co., Ltd.
+81 (0)3 3246 3155
Office of Public Relations, Strategic Planning Bureau
National Cancer Center
ncc-admin●ncc.go.jp
