Home > Information > press release > Questionable practices identified by an examination of therapeutic plan reviews performed by certified committees under the Act on the Safety of Regenerative Medicine
Questionable practices identified by an examination of therapeutic plan reviews performed by certified committees under the Act on the Safety of Regenerative Medicine
A research team led by Dr. Tsunakuni Ikka of the National Cancer Center Japan and Dr. Misao Fujita of Kyoto University highlights the independence, integrity, and quality of reviews of therapeutic plans for regenerative medicine.
The Act on the Safety of Regenerative Medicine (ASRM) was enacted in 2013 to ensure that regenerative medicine is accessible to the public in Japan in a safe and timely manner. Under the ASRM, before implementing regenerative medicine therapy or research, medical institutions must submit their plans for practicing regenerative medicine to a certified committee—Certified Committees (CCRM) or Certified Special Committees for Regenerative Medicine (CSCRM)—approved by the Minister of Health, Labour and Welfare (MHLW) for review. These collegial committees consist of experts in regenerative medicine, technology, law, and other related disciplines. CCRMs, with less stringent membership and expertise requirements, review the less risky Class III research and therapeutic plans. Conversely, the reviews of nominally riskier Classes II and I plans are performed by the more stringently defined CSCRMs.
Following the enactment of the ASRM, the quality of reviews conducted by the certified committees, which have significant legal implications, has been a focus of concerns raised by the Health Science Council (HSC), an advisory body to the MHLW. In response, MHLW commissioned a series of studies in 2019 to evaluate the reviews of research and therapeutic plans by the aforementioned certified committees.
In the commissioned studies, the researchers focused specifically on Class II therapeutic plans because of the higher risks associated with such interventions. They summarized the findings in the MHLW report and highlighted the implications and ongoing concerns for regulatory oversight of the provision of stem and other cell-based therapeutic interventions.
The following is a summary of the five studies conducted as part of their fact-finding investigation.
- The credibility of scientific evidence presented for therapeutic plan safety assessments
The researchers surveyed 351 therapeutic plans designated in the medium-risk (Class II) category and the 2,495 references they cited to determine whether they were sufficiently grounded by ample scientific evidence to ensure safe implementation. They identified plans that (1) did not reference any published work, (2) cited work from non-peer-reviewed media and/or unconfirmable sources, (3) cited articles published in so-called predatory journals, and (4) cited no clinical studies to demonstrate sufficient safety for therapeutic use. Altogether, 88 (25.1%) plans referred to questionable scientific evidence for proper safety assessment.
- The expertise of physicians involved in the therapeutic plans
The researchers surveyed 391 Class II proposals to examine whether the disease targeted by the proposed therapy matched the expertise of the physicians who would administer the treatment. Notably, they identified inconsistencies between the two in 117 CSCRM-reviewed plans (30.0%). Specifically, clear inconsistencies between the target indication and the provider scope of practice was identified in 55 proposals (14.1%). In the other 62 problematic plans (15.9%), the scope of practice for a given condition was unclear, or the relevant professional experience of the physicians could not be determined.
- Publicly available supporting documents associated with therapeutic plans
The researchers examined the titles of the proposals and the content of supporting documents from 371 Class II therapeutic plans. As a result, they learned that (1) 241 (65.0%) of the 371 therapeutic plans submitted for CSCRM review shared identical titles as other proposals, (2) the great majority of the plans submitted under identical titles included supporting materials that were essentially identical, except for minor modifications, and (3) in a smaller number of cases, an examination of the file properties of informed consent documents (.doc or .pdf) associated with multiple therapeutic plans revealed that a person or persons under the same username had prepared them.
These findings implied that many domestic institutions providing regenerative medicine therapies had duplicated and reused such supporting documents and therapeutic plans. Furthermore, (4) these duplicated therapeutic plans were often reviewed by the same handful of committees, suggesting they were specifically targeted to conduct reviews of questionable proposals.
- Tri-party relationships that do not warrant independent and impartial reviews
Through internet searches, the researchers confirmed four unidentified companies that linked institutions providing regenerative medicine-based therapies with CSCRMs. In other words, it was inferred that these companies would support a regenerative medicine practitioner by creating and implementing therapeutic plans, while on the other hand, are involved in the operation of specific CSCRMs and targets such committees to review the proposals submitted by the providers that the company supported (Figure). Under such tripartite relationships, the independent and fair reviews required by the ASRM are unlikely to have occurred.
- Website of regenerative medicine providers
The researchers surveyed 254 websites of regenerative medicine providers that implement Class II therapeutic plans to determine whether they contained any statements that could be categorized as exaggerated (false) advertising according to the Medical Care Act. As a result, 132 (51.9%) websites were found to contain suggestive statements such as “strict adherence to the ASRM procedures,” “approved by the MHLW,” or “reviewed by a nationally certified committee” to imply that the providers were subjected to heavy scrutiny by independent regulatory bodies.
The researchers hope that these findings will (1) generate social interest in the ongoing debate on the revision of the ASRM to ensure appropriate changes to the law to increase accountability and credibility to the operation of the review system and, (2) help patients understand more about regenerative medicine and make better treatment choices.
The results of this study were published online in Stem Cell Reports on Feb 23, 2023.
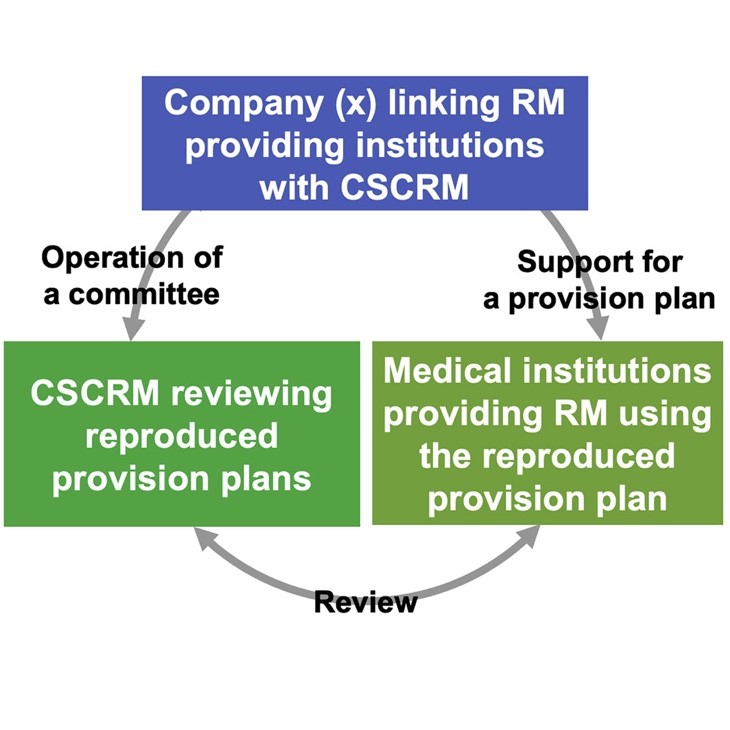
Figure Inappropriate three-way relationships that diminishes the independence and integrity of CSCRM reviews
Paper Details
Journal: Stem Cell Reports
Title: “Difficulties in ensuring review quality performed by committees under the Act on the Safety of Regenerative Medicine in Japan”
Authors: Tsunakuni Ikka*1,2, Misao Fujita*3,4, Taichi Hatta5, Tetsu Isobe6, Kenji Konomi7, Tatsuo Onishi8, Shoji Sanada9, Yuichiro Sato10, Shimon Tashiro11, Morikuni Tobita12
*:Corresponding authors
Author Affiliations:
- Division of Bioethics & Healthcare Law, Institute for Cancer Control, National Cancer Center Japan
- Division of Bioethics, Center for Research Administration and Support, National Cancer Center Japan
- Uehiro Research Division for iPS Cell Ethics, Center for iPS Cell Research and Application (CiRA), Kyoto University
- Institute for the Advanced Study of Human Biology (WPI-ASHBi), KUIAS, Kyoto University
- Graduate School of Public Health, Shizuoka Graduate University of Public Health
- Law School, Keio University
- Clinical and Translational Research Center, Keio University Hospital
- MLIP Law Office
- Clinical and Translational Research Center, Kobe University Hospital
- Faculty of Education, Tokyo Gakugei University
- Department of Sociology, Graduate School of Arts and Letters, Tohoku University,
- Medical Technology Innovation Center, Juntendo University
doi: https://doi.org/10.1016/j.stemcr.2023.01.013
