Home > Specialist ctrs & depts > Clinical Laboratories
Clinical Laboratories
Atsushi Ochiai, Takeshi Kuwata, Genichiro Ishii, Satoshi Fujii, Motohiro Kojima, Masato Sugano, Chisako Yamauchi, Eiichi Yoshikawa, Shigehisa Yoshida, Masahiro Inoue, Masahiro Karibe, Seiji Iwasaki, Miki Goto, Masaki Takeda, Satoru Sunohara, Hiromi Kimura, Yasuharu Hashimoto, Yukihiro Okano, Akiko Yamada, Mari Hisano, Mika Sasanuma, Aya Koike, Takuya Yamaguchi, Takuya Aiba, Keiko Nakai, Ayumi Setsuta, Mayumi Motohashi, Ayumi Nakanishi, Sayuri Shibayama, Izumi Suzuki, Yasuko Yoshihara, Kazumi Yamaguchi, Rie Taniguchi, Kumiko Sudo, Saki Nakamura, Kazuki Motohashi, Atsushi Watanabe, Eriko Iwamoto, Yasuteru Yamagishi, Kazumi Tamura, Asami Sekine, Nagisa Bouno, Rie Kuroiwa, Masayuki Ito, Michiko Iida, Yuki Soeda, Megumi Michikawa, Tomoko Seto, Emiko Yoshikawa, Yoshiko Ohtake, Miwa Yamada, Megumi Yamaguchi
Introduction
The Department of Pathology and Clinical Laboratories (DPCL) has two divisions: Pathology Division (PD) and Clinical Laboratory Division (CLD). Both divisions play a fundamental role in routine hospital service and support research activities at the National Cancer Center Hospital East (NCCHE).
DPCL received ISO15189:2007 accreditation in 2012, and successfully transited to the newest version (ISO15189:2012) in 2014, ensuring quality control and quality assurance of testing, including the one for clinical trials, performed in the departments. In 2015, two sections, Physiology and Supporting laboratory testing in clinical studies, received ISO15189:2012, ensuring the quality control and quality assurance of the testing, including the ones for clinical trials, performed in the departments with global standards.
Routine activities
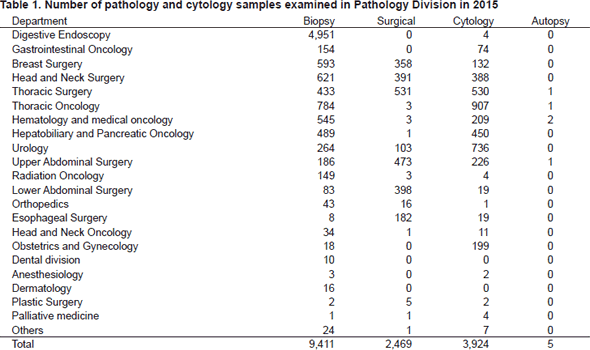
Primarily, the routine activity at the PD is surgical pathology. The Number of samples examined in the department in 2015 is listed in Table 1.
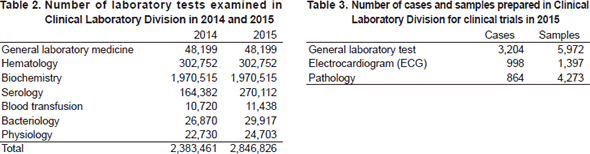
The CLD consists of seven sections: i) general laboratory medicine, ii) hematology, iii) biochemistry/serology, iv) Physiology, v) Bacteriology, vi) Blood transfusion and vii) Supporting laboratory tests in clinical studies. The numbers of tests performed in each division are listed in Table 2 and 3. The total number of tests performed in the DPCL in 2015 increased to 7.5% compared with the previous year; including a 94.4% and a 12.9% increase in the Blood transfusion and Serology sections, respectively.
Research activities
All of the pathologists were involved in research activities at RCIO (Research Center for Innovative Oncology). All the technologists working in the department are also highly motivated to develop advanced diagnostic technologies and various results are presented in several meetings.
Clinical trials
Practically, the CLD participated in all of the clinical trials operated at the NCCHE by providing laboratory data. The section for supporting laboratory testing in clinical studies was transferred to the DPCL in June 2014. The section, coordinating with the pathology and physiology sections, reinforces quality control and quality assurance for clinical tests performed in clinical trials at the NCCHE.
Education
Clinicopathological conferences are held regularly with each clinical department/section. In the PD, conference-style training sessions are open weekly for the residents.
Future prospects
Pathological diagnosis and laboratory tests play a fundamental role not only in routine hospital work but also in medical research. As an ISO15189-certified clinical laboratory, the DPCL will be continuously involved in investigating new diagnostic technologies, developing new drugs and conducting translational/clinical research in the NCCHE.