HOME > Publication & Reports > Annual Report 2016 > Center for Cancer Control and Information Services
Division of Medical Support and Partnership
Masashi Kato, Yasuaki Arai, Jun Itami, Nobuyoshi Hiraoka, Hiroshi Saito, Hironobu Hashimoto, Miki Hosoya, Toshiyuki Minemura, Yoko Nakazawa, Manami Fujishita, Ryoko Machii, Kayoko Kasuya, Kumiko Saika, Kazuko Matsuda, Hiroaki Onoya, Hideaki Kobayashi, Takashi Hanada, Yuichi Matsuyama, Miki Takahashi, Yoshiko Yamaya, Risa Hiranuma, Kaishi Satomi, Chieko Nagashima, Takatsugu Magara, Naoya Ikeno, Ritsuko Chinda, Mayumi Kobayashi, Mika Kobayashi, Hiroyo Ohchi, Hiromi Nakamura, Shiho Hirai
Introduction
The Division of Medical Support and Partnership builds partnerships with Designated Cancer Care Hospitals to support all health-allied professionals concerned with cancer control in Japan. The Medical Support and Partnership Section (MSPS) plays a unique role in supporting Designated Cancer Care Hospitals in Japan. The Pathology Consultation Section (PCS) makes effort to perform human pathology research based on the histology of tumor cells and tumor-stromal cells to improve diagnostic pathology of the tumors. The Radiology Consultation Section (RCS) provides a consultation service and a cancer image reference database (NCC-CIR). A radiology consultation service aims at the improvement of the quality of diagnosis based on medical images. The NCC-CIR is a web-based reference database system of images of neoplasms for physicians, radiologists, and pathologists, providing medical diagnostic images and information together with pathology.
The Outreach Radiation Oncology and Physics Section (ORPS) provides the following support programs for designated regional cancer centers and institutions participating in clinical trials. The Cancer Control Education and Training Section (CCETS) plays a central role in the planning, management, and evaluation of specialized and multidisciplinary training programs for physicians and other health professionals as trainers of each designated cancer care hospital, to promote a comprehensive and systematic cancer control program in Japan. The Cancer Screening Management Section (CSMS) supports the development of quality assurance management of cancer screening programs, particularly nationwide programs, which is based on the Basic Cancer Control Plan issued in 2007 and revised in 2012.
Our team and what we do
1.Networking among Designated Cancer Care Hospitals
The MSPS held the Designated Cancer Care Hospitals Liaison-council and the Palliative Care Committee to enhance partnerships for cancer control, and the PDCA cycle Forum to improve the quality of cancer care in Japan. The designated cancer care hospitals are important partners with the National Cancer Center (NCC) to promote comprehensive cancer control in Japan.
2.Pathology consultation service
The PCS received 469 cases requesting a specialist's second opinion regarding histopathological diagnosis in 2016. There are 88 consultants registered, many of them highly recognized experts in specialty disciplines. One of them assigned as a consultant examines the slides and quickly sends back his or her opinion report to each client. Most of the clients expressed satisfaction with the contents of the report and this consultation system. We also selected typical or educational cases from accumulated archives and constructed a referential database.
3.Radiology consultation service
Twenty-eight consultation reports have been put together for requests mainly from the Kanto and Chugoku regions. Hepato-biliary-pancreatic and musculoskeletal lesions were the common subjects. Consultation with a specialist was the most frequent reason (36.9%) for consultation. The client radiologists have evaluated 493 (91.3%) of the 540 consultation reports as being useful for the presence of a clinical impact on the final radiological diagnoses.
4.NCC-CIR
The average number of effective accesses to this site was almost the same as that in 2015, about 100,000 per month, with 301 cases with cancers shown.
5.Radiotherapy case service
Mailed dosimetry and on-site dosimetry were performed in 104 institutions and 14 institutions, respectively, at the ORPS. All data of the institutions were within the permissible limit.
6.Quality assurance (QA) activities in cancer screening
1)The CSMS collected the information related to the implementation of cancer screening and its management situation using Checklists (CLs) as a structure indicator in quality assurance (QA) at municipalities. The CSMS also evaluated process indicators such as rate of work-up, and ranked those indicators in all cities by prefecture in order of goodness so that each city compares its indicator with those of other cities.
2)The CSMS examined the method of activating QA by prefectural initiative, and set up the web site as a tool which supports prefectural activity. In 2016, prefecture-based data base about CLs and process indicators, and the manual of QA for prefectural staff were placed on the website.
Research activities
1.Development of a method to implement a PDCA cycle among the Designated Cancer Care Hospitals
The MSPS developed a method to implement the PDCA cycle continuously in prefectures.
2.Development of the IMRT quality control support program
The ORPS were developing enforcement of the mailed dosimetry regarding the output dose of Intensity Modulated Radiotherapy (IMRT) in five institutions (designated regional cancer centers).
3.Nationwide survey of the methods for improving Quality assurance (QA) in cancer screening
The CSMS investigated the concrete method to improve QA and collected barriers and effective examples for attaining CLs from the hearing survey to municipalities. These cases will be offered in 2017 from the website mentioned above.
Clinical trials
1.The on-site dosimetry regarding the output dose of IMRT
In the Japan Clinical Oncology Group (JCOG 1008, JCOG 1208, JCOG 1212, JCOG 1303, JCOG 1408) and the Japanese Radiation Oncology Study Group (JROSG 12-1), the ORPS performed on-site dosimetry regarding the output dose of IMRT in nine institutions.
2.Support for clinical trials
To support central radiological review in clinical trials, we have provided a system for receiving and sending DICOM imaging data between participating multi-centers and review board since 2014. Six clinical trials used this system in 2016.
Education
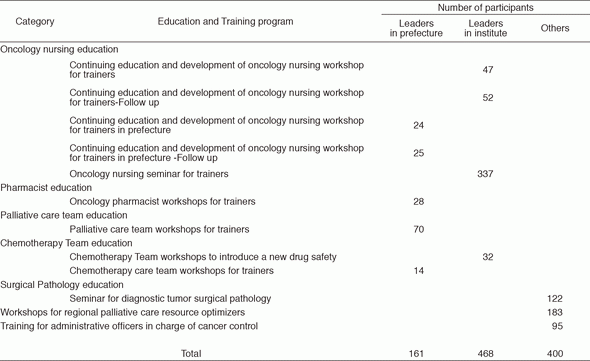
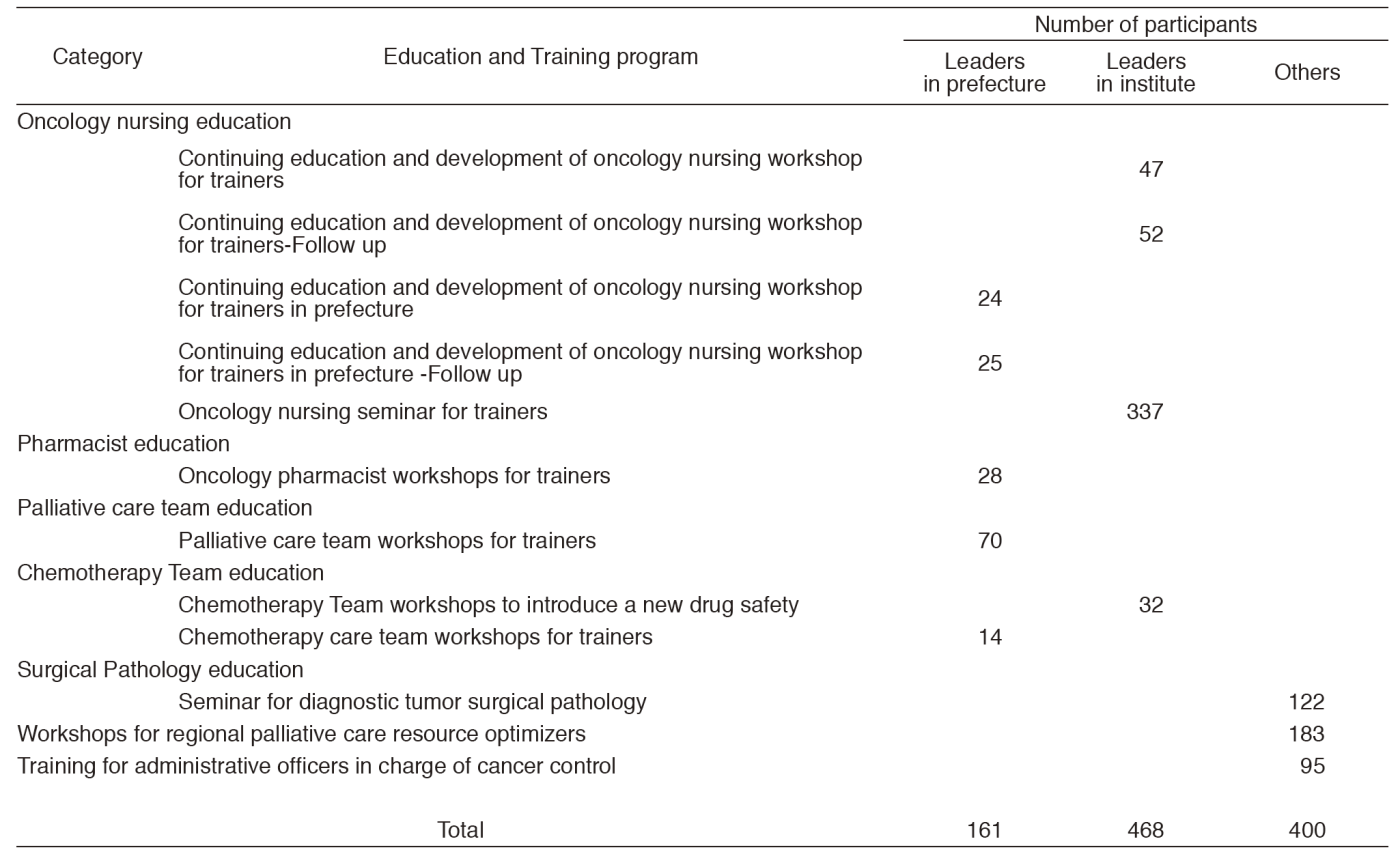
The CCETS provides and evaluates various oncology professional training programs about information on diagnosis, chemotherapy, palliative care, nursing care for physicians, nurses, pharmacists, and so on. The CCETS provides multidisciplinary training programs for Palliative Care Teams and Chemotherapy Teams. In order to develop leaders in each prefecture, the CCETS holds leadership training and follow-up programs for skilled physicians, nurses, and pharmacists (Table 1).
The CSMS held one-day educational workshops twice for the members of prefectural committees and administrative staff of cancer screening management, aiming at activating QA activities in each of 47 prefectures. There were 135 participants in the workshops from 45 prefectures.
Future prospects
The MSPS searches a support system to meet the needs of Designated Cancer Care Hospitals and cancer screening management at municipalities. All sections will continue to be involved in our routine activities and education.
List of papers published in 2016
Journal
1.Nakazawa Y, Kato M, Yoshida S, Miyashita M, Morita T, Kizawa Y. Population-Based Quality Indicators for Palliative Care Programs for Cancer Patients in Japan: A Delphi Study. J Pain Symptom Manage, 51:652-661, 2016
2.Sekiguchi M, Igarashi A, Matsuda T, Matsumoto M, Sakamoto T, Nakajima T, Kakugawa Y, Yamamoto S, Saito H, Saito Y. Optimal use of colonoscopy and fecal immunochemical test for population-based colorectal cancer screening: a cost-effectiveness analysis using Japanese data. Jpn J Clin Oncol, 46:116-125, 2016
3.Hirai K, Ishikawa Y, Fukuyoshi J, Yonekura A, Harada K, Shibuya D, Yamamoto S, Mizota Y, Hamashima C, Saito H. Tailored message interventions versus typical messages for increasing participation in colorectal cancer screening among a non-adherent population: A randomized controlled trial. BMC Public Health, 16:431, 2016
4.Machii R, Saika K. Subsite distribution of stomach cancer from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol, 46:98, 2016
5.Saika K, Machii R. Subsite distribution of colon cancer from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol, 46:190, 2016
6.Saika K, Machii R. Incidence rate for prostate cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:1074, 2016
7.Young GP, Senore C, Mandel JS, Allison JE, Atkin WS, Benamouzig R, Bossuyt PMM, Silva MD, Guittet L, Halloran SP, Haug U, Hoff G, Itzkowitz SH, Leja M, Levin B, Meijer GA, O'Morain CA, Parry S, Rabeneck L, Rozen P, Saito H, Schoen RE, Seaman HE, Steele RJC, Sung JJY, Winawer SJ. Recommendations for a step-wise comparative approach to the evaluation of new screening tests for colorectal cancer. Cancer, 122:826-839, 2016
8.Machii R, Saika K. Incidence rate for liver cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:1181-1182, 2016
9.Hamashima C, Hamashima C C, Hattori M, Honjo S, Kasahara Y, Katayama T, Nakai M, Nakayama T, Morita T, Ohta K, Ohnuki K, Sagawa M, Saito H, Sasaki S, Shimada T, Sobue T, Suto A. The Japanese Guidelines for Breast Cancer Screening. Jpn J Clin Oncol, 46:482-492, 2016
10.Miyoshi Y, Yorifuji T, Horikawa R, Takahashi I, Nagasaki K, Ishiguro H, Fujiwara I, Ito J, Oba M, Kawamoto H, Fujisaki H, Kato M, Shimizu C, Kato T, Matsumoto K, Sago H, Takimoto T, Okada H, Suzuki N, Yokoya S, Ogata T, Ozono K. Gonadal function, fertility, and reproductive medicine in childhood and adolescent cancer patients: a national survey of Japanese pediatric endocrinologists. Clin Pediatr Endocrinol, 25:45-57, 2016
11.Hori M, Onaya H, Hiraoka N, Yamaji T, Kobayashi H, Takahashi M, Mutoh M, Shimada K, Nakagama H. Evaluation of the degree of pancreatic fatty infiltration by area-based assessment of CT images: comparison with histopathology-based and CT attenuation index-based assessments. Jpn J Radiol, 34:667-676, 2016
12.Ohashi M, Morita S, Fukagawa T, Wada T, Kushima R, Onaya H, Katai H. Evaluation of 64-Channel Contrast-Enhanced Multi-detector Row Computed Tomography for Preoperative N Staging in cT2-4 Gastric Carcinoma. World J Surg, 40:165-171, 2016
13.Sakashita A, Kishino M, Nakazawa Y, Yotani N, Yamaguchi T, Kizawa Y. How to Manage Hospital-Based Palliative Care Teams Without Full-Time Palliative Care Physicians in Designated Cancer Care Hospitals: A Qualitative Study. Am J Hosp Palliat Care, 33:520-526, 2016
14.Nakamura M, Minemura T, Ishikura S, Nishio T, Narita Y, Nishimura Y. An on-site audit system for dosimetry credentialing of intensity-modulated radiotherapy in Japanese Clinical Oncology Group (JCOG) clinical trials. Phys Med, 32:987-991, 2016