HOME > Publication & Reports > Annual Report 2016 > Center for Cancer Control and Information Services
Center for Cancer Registries
Hiroshi Nishimoto, Naoyuki Sato, Tomohiro Matsuda, Kota Katanoda, Akiko Shibata, Kumiko Saika, Megumi Hori, Mariko Niino, Ayako Okuyama, Toru Sagami, Yoshiko Emori, Kaori Nakano, Rika Nabata, Mika Mizuochi, Saya Maruyama, Seiya Kondo, Hiroko Takamatsu, Asako Umeda, Tokiko Iguchi, Hiroko Okazaki, Masako Hoshikawa
Introduction
The Center for Cancer Registries is in charge of providing precise cancer statistics for patients and their families, the public, healthcare professionals, policy makers, and researchers. Our center promotes nationwide cancer control programs based on the newly enacted Act on Promotion of Cancer Registries, as well as standardization of hospital-based cancer registries in designated cancer care hospitals.
Our team and what we do
1.Population-based Cancer Registries
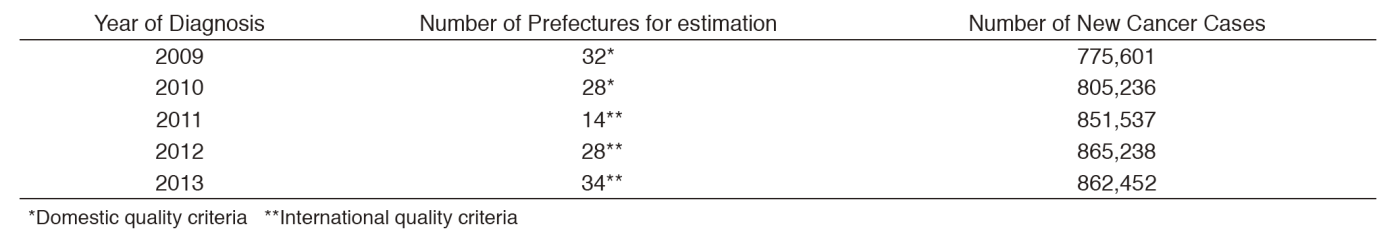
Based on the Act on Promotion of Cancer Registries, we collect cancer information from 47 prefectures by using the online National Cancer Registry System (NCRS). We have continuously exerted efforts to develop a reliable cancer surveillance system in Japan, which is stated as a key element in the Cancer Control Act. We support all 47 prefectures by disseminating up-to-date information through websites and mailing lists; by setting up a Q&A service; by holding 2-day educational workshops for cancer registrars and administrative officers in charge of cancer control in May and December. We also conduct site visits for onsite training. The Population-Based Cancer Registry Database System (PBCRDS), developed by our center, allows prefectures to maintain cancer registry data in the past years, and to link them with the current data in the NCRS. Forty five registries have introduced the PBCRDS as of January 2017. An external audit on security control in cancer registration was performed in collaboration with the professional agency. Our regular activities include a consulting service for the Ministry of Health, Labour and Welfare, and for the related boards.
2.Hospital-based Cancer Registries
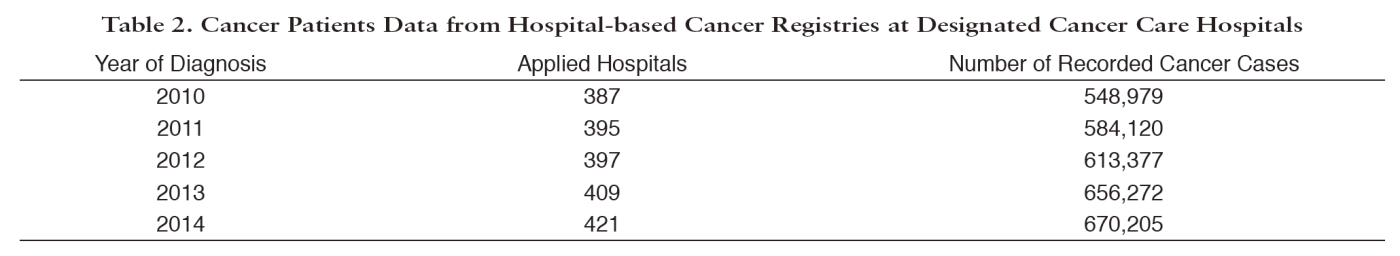
Since Hospital-Based Cancer Registry (HBCR) is essential to evaluate cancer care in each hospital and also to achieve high completeness of PBCR, we examine the quality of HBCR data. The collected HBCR data are analyzed to calculate clinical cancer statistics. We play an important role as a driving force for the standardization and quality improvement of HBCR, which was performed at 433 designated cancer care hospitals (DCCHs) and over 318 other hospitals, including 15 designated hospitals for childhood cancer, in 2016. Individual records for 670,205 cancer cases diagnosed in 2014 were collected from 421 DCCHs. In collaboration with experts, we developed the standards for HBCR, and distributed the standardized software "Hos-CanR Plus", which is introduced in about 1,400 hospitals, and the simplified version, "Hos-CanR Lite" as well. To improve data quality, we organized a comprehensive education program for cancer registrars through holding 29 workshops in several levels in 2016, and conducted a qualification test for certifying cancer registrars.
3.Cancer Statistics
The Center for Cancer Registries is in charge of providing information on cancer statistics; the updated data of cancer mortality in 2015, incidence in 2012, and survival in 2006-8.
Research activities
The national cancer incidences in 2013 were estimated based on data from all 47 cancer registries, as 862,452. Thirty-four prefectures have met the data quality standards. Incidence data were then analyzed in detail by cancer site, and published in international academic journals, presented at conferences both in Japan and abroad, on the web site and in the book titled "Cancer Statistics in Japan".
As a routine activity, international comparisons of cancer burden and survival rate were conducted based on the WHO mortality, GLOBOCAN, and cancer registry database. Updated trend analysis of cancer incidence and mortality in Japan was conducted.
Education
Our activities of extramural education were executed as mentioned in the strand of "Our team and what we do".
Future prospects
The National Cancer Registry (NCR) was launched in January 2016. We set the activities on a better track, by restructuring our center, and by building a new relationship with the International Agency of Research on Cancer (IARC) and other overseas institutions.
List of papers published in 2016
Journal
1.Hori M, Katanoda K. Incidence rate for uterus cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:970-971, 2016
2.Katanoda K. Neuroblastoma Mass Screening--What Can We Learn From It? J Epidemiol, 26:163-165, 2016
3.Katanoda K, Hori M. Incidence rate for breast cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:883, 2016
4.Matsuda T, Niino M. Type distribution of lymphoid leukemia from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol, 46:290, 2016
5.Machii R, Saika K. Subsite distribution of stomach cancer from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol, 46:98, 2016
6.Saika K, Machii R. Subsite distribution of colon cancer from Cancer Incidence in Five Continents Vol. X. Jpn J Clin Oncol, 46:190, 2016
7.Saika K, Matsuda T. Cancer incidence rate in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:495-496, 2016
8.Okuyama A, Saika K. Incidence rates for colon cancer of Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:792-793, 2016
9.Saika K, Machii R. Incidence rate for prostate cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:1074, 2016
10.Machii R, Saika K. Incidence rate for liver cancer in Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:1181-1182, 2016
11.Niino M, Okuyama A. Incidence rates for lung and bronchus cancer of Japanese in Japan and in the United States from the Cancer Incidence in Five Continents. Jpn J Clin Oncol, 46:698-699, 2016
12.Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K. Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol, 46:942-951, 2016
13.Katanoda K, Kamo K, Tsugane S. Quantification of the increase in thyroid cancer prevalence in Fukushima after the nuclear disaster in 2011--a potential overdiagnosis? Jpn J Clin Oncol, 46:284-286, 2016
14.Takaoka Md M, Okuyama A, Mekata E, Masuda M, Otani M, Higashide S, Higashi T. Staging discrepancies between Hospital-Based Cancer Registry and Diagnosis Procedure Combination data. Jpn J Clin Oncol, 46:788-791, 2016
15.Saito E, Charvat H, Goto A, Matsuda T, Noda M, Sasazuki S, Inoue M. Burden of cancer associated with type 2 diabetes mellitus in Japan, 2010-2030. Cancer Sci, 107:521-527, 2016