HOME > Publication & Reports > Annual Report 2016 > Hospital East
Department of Breast and Medical Oncology
Hirofumi Mukai, Yoichi Naito, Nobuaki, Matsubara, Takahiro Kogawa, Masaoki Sasaki, Kenichi Harano, Ako Hosono, Mai Onomura, Yoko Yamada, and Mayuko Ito, Yumi Fujimoto, Yoriko Hasegawa, Yujiro Ueda, Takaaki Yokoyama, Tetsuya Urasaki
Clinical and research activities
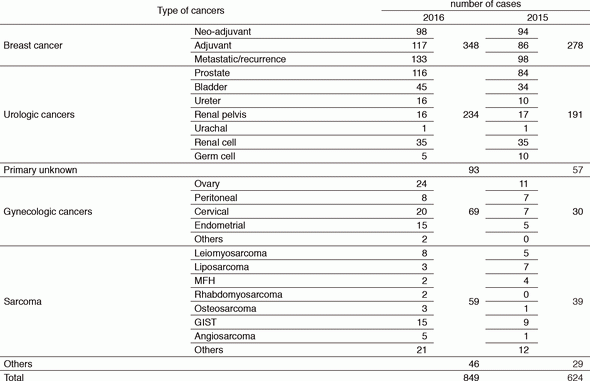
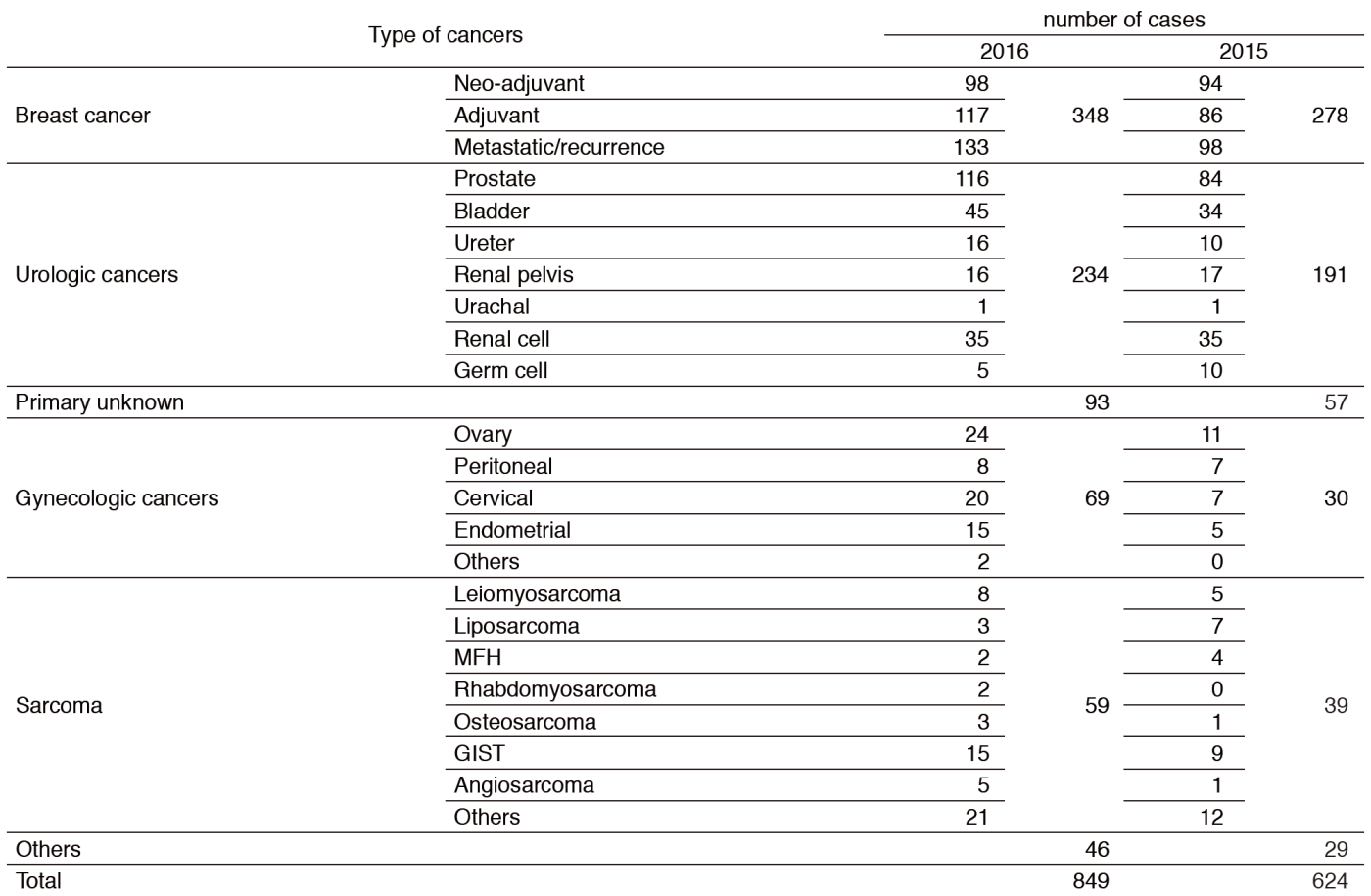
We take care of patients with breast cancer, urologic and gynecologic cancers, sarcoma, pediatric cancers, and many other malignancies, including rare cancers that are difficult to be treated at other hospitals because of the absence of standard therapy. The number of new patients in 2016 substantially increased compared to the year 2015 (Table 1). It was possibly because we had built closer and visible partnership with local medical facilities and we had developed systems to share information about clinical trials of investigational new drugs.
We are performing not only retrospective studies taking advantage of high number of cases, but also prospective clinical trials, developmental clinical trials, and their associated translational researches. Further, we are exploring response-predictive and prognostic biomarkers through immune-function analysis and genomic analysis.
Research achievement
-We enrolled 35 patients to industry-initiated clinical trials.
-We are enrolling patients to an investigator-initiated trial, RESET trial.
-We were commended by the CSPOR-BC (The Comprehensive Support Project for Oncological Research of Breast Cancer) for enrolling the second most patients among all participating institutes.
-We conducted multi-center trials through the JCOG (The Japan Clinical Oncology Group) and other trial groups and enrolled patients.
-We are implementing AMED-supported trials, "Strategy for neoadjuvant systemic therapy in breast cancer" (PI, Mukai H) and "Project for diagnosis of super-high risk group by genomic analysis and construction of evidence for stratified/individualized prophylaxis" (sub-investigator, Naito Y).
Education
Our goal in education is to foster "genuine" medical oncologists. Graduates from our Resident/Chief Resident Programs are expected to become able to provide not only standard therapies regardless of types of cancer, but also multi-disciplinary care cooperating with other medical professionals. Obtaining skills for palliative care and dealing with oncologic emergencies are also expected. Further, we require and help them to implement clinical research to address clinical questions they find for themselves, and report the results on internal journals. That is because we want to develop scientific clinicians who can focus on issues scientifically and create evidence for themselves.
Future prospects
In clinic, we will provide clinical care with high patients'satisfaction through multi-disciplinary team approach. In education, we will foster medical oncologists who have skill and knowledge for cross-organ oncology care and scientific acumen. In research, we will lead multi-center clinical trials and phases I to III developmental trials of investigational drugs. Further, we will expand our activity to pre-clinical research for predicting response and overcoming resistance to anti-cancer drugs.
List of papers published in 2016
Journal
1.Harano K, Yonemori K, Hirakawa A, Shimizu C, Katsumata N, Gemma A, Fujiwara Y, Tamura K. The influence of familial factors on the choice of the place of death for terminally ill breast cancer patients: a retrospective single-center study. Breast Cancer, 23:797-806, 2016
2.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Prognostic factors in patients with uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Int J Clin Oncol, 21:168-176, 2016
3.Komai Y, Sugimoto M, Gotohda N, Matsubara N, Kobayashi T, Sakai Y, Shiga Y, Saito N. Patient-specific 3-dimensional Printed Kidney Designed for "4D" Surgical Navigation: A Novel Aid to Facilitate Minimally Invasive Off-clamp Partial Nephrectomy in Complex Tumor Cases. Urology, 91:226-233, 2016
4.Niikura N, Ota Y, Hayashi N, Naito M, Kashiwabara K, Watanabe K, Yamashita T, Mukai H, Umeda M. Evaluation of oral care to prevent oral mucositis in estrogen receptor-positive metastatic breast cancer patients treated with everolimus (Oral Care-BC): randomized controlled phase III trial. Jpn J Clin Oncol, 46:879-882, 2016
5.Ishikawa T, Sakamaki K, Narui K, Kaise H, Tsugawa K, Ichikawa Y, Mukai H. Prospective cohort study of febrile neutropenia in breast cancer patients with neoadjuvant and adjuvant chemotherapy: CSPOR-BC FN study. Jpn J Clin Oncol, 46:692-695, 2016
6.Tozaki M, Kuroki Y, Kikuchi M, Kojima Y, Kubota K, Nakahara H, Ito Y, Mukai H. The Japanese Breast Cancer Society clinical practice guidelines for screening and imaging diagnosis of breast cancer, 2015 edition. Breast Cancer, 23:357-366, 2016
7.Jinno H, Inokuchi M, Ito T, Kitamura K, Kutomi G, Sakai T, Kijima Y, Wada N, Ito Y, Mukai H. The Japanese Breast Cancer Society clinical practice guideline for surgical treatment of breast cancer, 2015 edition. Breast Cancer, 23:367-377, 2016
8.Horii R, Honma N, Ogiya A, Kozuka Y, Yoshida K, Yoshida M, Horiguchi S, Ito Y, Mukai H. The Japanese Breast Cancer Society clinical practice guidelines for pathological diagnosis of breast cancer, 2015 edition. Breast Cancer, 23:391-399, 2016
9.Aihara T, Toyama T, Takahashi M, Yamamoto Y, Hara F, Akabane H, Fujisawa T, Ishikawa T, Nagai S, Nakamura R, Tsurutani J, Ito Y, Mukai H. The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer, 2015 edition. Breast Cancer, 23:329-342, 2016
10.Yamauchi C, Sekiguchi K, Nishioka A, Arahira S, Yoshimura M, Ogo E, Oguchi M, Ito Y, Mukai H. The Japanese Breast Cancer Society Clinical Practice Guideline for radiation treatment of breast cancer, 2015 edition. Breast Cancer, 23:378-390, 2016
11.Taira N, Arai M, Ikeda M, Iwasaki M, Okamura H, Takamatsu K, Nomura T, Yamamoto S, Ito Y, Mukai H. The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2015 edition. Breast Cancer, 23:343-356, 2016
12.Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, Park Y, Toyama T, Hozumi Y, Tsurutani J, Imoto S, Watanabe T, Sagara Y, Nishimura R, Shimozuma K, Ohashi Y. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol, 17:90-98, 2016
13.Mukai H, Arihiro K, Shimizu C, Masuda N, Miyagi Y, Yamaguchi T, Yoshida T. Stratifying the outcome after neoadjuvant treatment using pathological response classification by the Japanese Breast Cancer Society. Breast Cancer, 23:73-77, 2016
14.Yamada Y, Matsubara N, Tabata K, Satoh T, Kamiya N, Suzuki H, Kawahara T, Uemura H, Yano A, Kawakami S. Abiraterone acetate after progression with enzalutamide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer: a multi-center retrospective analysis. BMC Res Notes, 9:471, 2016
15.Mukai H, Kato K, Esaki T, Ohsumi S, Hozomi Y, Matsubara N, Hamaguchi T, Matsumura Y, Goda R, Hirai T, Nambu Y. Phase I study of NK105, a nanomicellar paclitaxel formulation, administered on a weekly schedule in patients with solid tumors. Invest New Drugs, 34:750-759, 2016
16.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Optimal cytoreductive surgery in patients with advanced uterine carcinosarcoma: A multi-institutional retrospective study from the Japanese gynecologic oncology group. Gynecol Oncol, 141:447-453, 2016
17.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
18.Harano K, Lei X, Gonzalez-Angulo AM, Murthy RK, Valero V, Mittendorf EA, Ueno NT, Hortobagyi GN, Chavez-MacGregor M. Clinicopathological and surgical factors associated with long-term survival in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat, 159:367-374, 2016
19.Kogawa T, Fouad TM, Liu DD, Wu J, Shen Y, Masuda H, Fujii T, Chavez-MacGregor M, Alvarez RH, Hortobagyi GN, Valero V, Ueno NT. High HER2/Centromeric Probe for Chromosome 17 Fluorescence In Situ Hybridization Ratio Predicts Pathologic Complete Response and Survival Outcome in Patients Receiving Neoadjuvant Systemic Therapy With Trastuzumab for HER2-Overexpressing Locally Advanced Breast Cancer. Oncologist, 21:21-27, 2016
20.Doi A, Sumiyoshi T, Omori Y, Oyamada Y, Kumano K, Yoshizaki N, Hirayama M, Suzuki Y, Okushiba S, Kogawa T, Doi T, Kondo H. Double Extramedullary Plasmacytoma of the Stomach with a Long-term Endoscopic Follow-up. Intern Med, 55:3585-3590, 2016
21.Yoshino T, Kojima T, Bando H, Yamazaki T, Naito Y, Mukai H, Fuse N, Goto K, Ito Y, Doi T, Ohtsu A. Effect of food on the pharmacokinetics of TAS-102 and its efficacy and safety in patients with advanced solid tumors. Cancer Sci, 107:659-665, 2016
22.Mukai H, Saeki T, Aogi K, Naito Y, Matsubara N, Shigekawa T, Ueda S, Takashima S, Hara F, Yamashita T, Ohwada S, Sasaki Y. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci, 107:1465-1470, 2016
23.Mukai H, Higashi T, Sasaki M, Sobue T. Quality evaluation of medical care for breast cancer in Japan. Int J Qual Health Care, 28:110-113, 2016