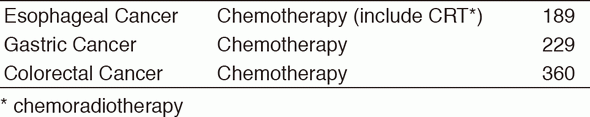HOME > Publication & Reports > Annual Report 2016 > Hospital East
Department of Gastrointestinal Oncology
Takayuki Yoshino, Toshihiko Doi, Takashi Kojima, Kouhei Shitara, Hideaki Bando, Yasutoshi Kuboki, Nozomu Fuse, Shota Fukuoka, Akihito Kawazoe, Kentaro Sawada
Introduction
In 2016, approximately 780 gastrointestinal (GI) cancer patients were treated by staff oncologists and skilled residents in the Department of GI Oncology, which focuses on the optimal chemotherapy W/ or W/O radiation for the treatment of GI cancers.
Our team and what we do
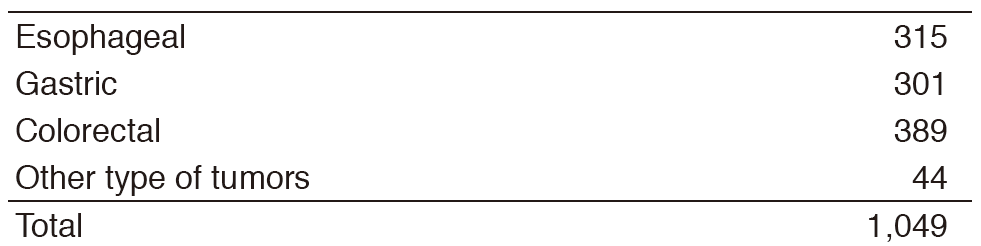
Inter-Divisional tumor board conferences with the Surgical/Radiation Oncology Divisions are held regularly to review the current treatment for each patient and to discuss further treatment strategies. Basically, routine chemotherapy is done on an outpatient basis, and there are approximately 1,900 selected patients who need hospitalization for the purpose of planned therapy with chemotherapy or palliation. Our activities for each type of GI cancer in 2016 are shown in Table 1 (Number) and Table 2 (Treatment). There are ongoing clinical trials which are consisted of 49 Phase I trials including globally first-in-class (FIC), first-in-human (FIH), investigational new drugs (INDs), and 32 Phase II/III clinical trials in order to approve the INDs.
Research activities
1. Phase I
Our Department has focused more on early stage clinical development of INDs. The number of patients enrolled for phase I trials has been increasing recently, especially in studies for immunomodulating agents. Several results of phase I trials, such as new HER2 ADC (DS-8201a), TAS-116, a novel Hsp90 inhibitor, and TAS-114, a dUTPase inhibitor in combination with S-1 were published or presented at international meetings. Phase II study of TAS-116 for GIST is ongoing. In addition, MSB0011359C (Anti-PD-L1/TGFbetaRII) has expanded to target for gastric and esophageal cancer. MK-3475 basket trial for MSI-H cancer without colorectal cancer is ongoing.
2. Esophageal Cancer (EC)
As a basic research, we presented a comprehensive immunohistochemically analysis of tumor microenvironment immune status in ESCC, and a prognostic significance of tumor regression grade for patients with ESCC. A multicenter phase I study of HSP105-derived peptide vaccine for patients with advanced esophageal cancer/ colorectal cancer, and phase II trial of BKM120 in patients with advanced esophagus cancer were enrolled. As in the single institutional clinical study, Phase I trial of definitive chemoprotontherapy in patients with clinical stage I/II/III esophageal carcinoma is ongoing. Phase I trial of oncolytic virus and pembrolizumab will be started.
3. Gastric Cancer (GC)
An investigator-initiated trial of phase I trial of sulfasalazine (SSZ), which targets cancer stem-like cell fraction, plus cisplatin for CD44v gastric cancer which refractory to cisplatin has finished its enrollment. Since September 2015, we have initiated an immune monitoring study to evaluate several immunological properties such as classification of lymphocyte or expression of immune checkpoint in tumor infiltrating lymphocyte, and PBMC before and after treatment, which will hopefully lead to personalized therapy in the field of immunotherapy. We will present clinicopathological analysis of PDL1 expression, microsatellite instability, EB virus status and its correlation with outcome of gastric cancer in the near future. Also, results of change of regulatory-T cells fraction in tumor infiltrating lymphocytes after ramucirumab treatment will be presented in upcoming meetings. Phase I trial of regorafenib and nivolumab, or anti-CD4 depleting antibody IT1208 will be started soon for solid tumors including gastric cancer.
4. Colorectal Cancer (CRC)
We have established the SCRUM-Japan GI-SCREEN 2013-01-CRC (UMIN000016343). We also started GI-SCREEN CRC-MSI, which is the multi-center project for screening the microsatellite instability (MSI) status of Japanese CRC patients. Based on the screening system of GI-SCREEN, the investigator-initiated clinical trial (IIT) for metastatic CRC patients with HER2 amplifications will soon be started and the IIT for patients with BRAF mutations will be planned. The clinical evaluation studies of cell-free DNA-based RAS gene testing by using BEAMing technology, and of newly developed MSI testing kits are currently conducted.
We have completed the phase Ib/II trial of the novel combination of TAS-102 plus bevacizumab as an investigator-initiated trial (IIT). The patients'recruitments of the phase Ib/II trial of the novel combination of TAS-102 plus nintedanib were completed. Currently, phase Ib/II trial of the combination of BBI-608 plus pembrolizumab for metastatic CRC patients, and phase Ib/II trial of chemoradiation followed by nivolumab and surgery for resectable CRC patients are ongoing.
Education
Our residents learned the latest evidence-based medicine and applied this knowledge pragmatically to enhance care for patients with GI cancers, and eventually acquire qualifications as a comprehensive GI oncologist through daily practice and direct training from our staff. Accordingly, our staff actively provide a pile of valuable opportunities to polish their skills regarding various chemotherapies, especially in collaboration with the Department of Experimental Therapeutics, as well as diagnostic & therapeutic endoscopies collaborated with the Department of Digestive Endoscopy. We regularly hold tumor board meetings and frequently have numerous face-to-face opportunities with experts in different specialties. We instruct them how to conduct valuable clinical trials, how to have the chance to attend international academic conferences, and the best way to present the academic meeting and work on many high-impact articles in scholarly journals. To date, our department has led many residents to become 'true'skilled GI oncologists who play major roles at leading cancer centers across the country.
Future prospects
We continue to provide the best treatment for cancer patients, the best education for residents, and aim to perform the following activities:
1)To provide more of the latest, cutting-edged medicine to cancer patients, and to foster more of the next generation of skilled GI oncologists.
2)To achieve medical innovation from Japan, we aim to play leading roles in the clinical developments of INDs by contributing to various types of clinical trials including FIC, FIH early trials, IITs with proof-of-concept, and international clinical trials.
3)To enhance our research activity, we will establish research networks with cutting-edged researchers in Japan as well as globally.
List of papers published in 2016
Journal
1.Shitara K, Muro K, Shimada Y, Hironaka S, Sugimoto N, Komatsu Y, Nishina T, Yamaguchi K, Segawa Y, Omuro Y, Tamura T, Doi T, Yukisawa S, Yasui H, Nagashima F, Gotoh M, Esaki T, Emig M, Chandrawansa K, Liepa AM, Wilke H, Ichimiya Y, Ohtsu A. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer, 19:927-938, 2016
2.Bando H, Takebe N. Perspectives on research activity in the USA on Cancer Precision Medicine. Jpn J Clin Oncol, 46:106-110, 2016
3.Bando H, Yamada Y, Tanabe S, Nishikawa K, Gotoh M, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I. Efficacy and safety of S-1 and oxaliplatin combination therapy in elderly patients with advanced gastric cancer. Gastric Cancer, 19:919-926, 2016
4.Yoshino T, Uetake H, Fujita N, Furuta T, Katori J, Hara N, Muro K. TAS-102 Safety in Metastatic Colorectal Cancer: Results From the First Postmarketing Surveillance Study. Clin Colorectal Cancer, 15:e205-e211, 2016
5.Hamada C, Yamada Y, Azuma M, Nishikawa K, Gotoh M, Bando H, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hyodo I. Meta-analysis supporting noninferiority of oxaliplatin plus S-1 to cisplatin plus S-1 in first-line treatment of advanced gastric cancer (G-SOX study): indirect comparison with S-1 alone. Int J Clin Oncol, 21:668-675, 2016
6.Kanai M, Kawaguchi T, Kotaka M, Shinozaki K, Touyama T, Manaka D, Ishigure K, Hasegawa J, Munemoto Y, Matsui T, Takagane A, Ishikawa H, Matsumoto S, Sakamoto J, Saji S, Yoshino T, Ohtsu A, Watanabe T, Matsuda F. Large-scale prospective pharmacogenomics study of oxaliplatin-induced neuropathy in colon cancer patients enrolled in the JFMC41-1001-C2 (JOIN Trial). Ann Oncol, 27:1143-1148, 2016
7.Obermannova R, Van Cutsem E, Yoshino T, Bodoky G, Prausova J, Garcia-Carbonero R, Ciuleanu T, Garcia Alfonso P, Portnoy D, Cohn A, Yamazaki K, Clingan P, Lonardi S, Kim TW, Yang L, Nasroulah F, Tabernero J. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol, 27:2082-2090, 2016
8.Ushijima T, Yoshino T. The Moment that KRAS Mutation Started to Evolve into Precision Medicine in Metastatic Colorectal Cancer. Cancer Res, 76:6443-6444, 2016
9.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Kohne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Osterlund P, Oyen WJG, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taieb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol, 27:1386-1422, 2016
10.Van Cutsem E, Yoshino T, Hocke J, Oum'Hamed Z, Studeny M, Tabernero J. Rationale and Design for the LUME-Colon 1 Study: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Nintedanib Plus Best Supportive Care Versus Placebo Plus Best Supportive Care in Patients With Advanced Colorectal Cancer Refractory to Standard Treatment. Clin Colorectal Cancer, 15:91-94 e91, 2016
11.Kuboki Y, Yamashita S, Niwa T, Ushijima T, Nagatsuma A, Kuwata T, Yoshino T, Doi T, Ochiai A, Ohtsu A. Comprehensive analyses using next-generation sequencing and immunohistochemistry enable precise treatment in advanced gastric cancer. Ann Oncol, 27:127-133, 2016
12.Yamanaka T, Oki E, Yamazaki K, Yamaguchi K, Muro K, Uetake H, Sato T, Nishina T, Ikeda M, Kato T, Kanazawa A, Kusumoto T, Chao C, Lopatin M, Krishnakumar J, Bailey H, Akagi K, Ochiai A, Ohtsu A, Ohashi Y, Yoshino T. 12-Gene Recurrence Score Assay Stratifies the Recurrence Risk in Stage II/III Colon Cancer With Surgery Alone: The SUNRISE Study. J Clin Oncol, 34:2906-2913, 2016
13.Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer, 19:183-191, 2016
14.Hironaka S, Sugimoto N, Yamaguchi K, Moriwaki T, Komatsu Y, Nishina T, Tsuji A, Nakajima TE, Gotoh M, Machida N, Bando H, Esaki T, Emi Y, Sekikawa T, Matsumoto S, Takeuchi M, Boku N, Baba H, Hyodo I. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncol, 17:99-108, 2016
15.Zenda S, Kojima T, Kato K, Izumi S, Ozawa T, Kiyota N, Katada C, Tsushima T, Ito Y, Akimoto T, Hasegawa Y, Kanamaru M, Daiko H. Multicenter Phase 2 Study of Cisplatin and 5-Fluorouracil With Concurrent Radiation Therapy as an Organ Preservation Approach in Patients With Squamous Cell Carcinoma of the Cervical Esophagus. Int J Radiat Oncol Biol Phys, 96:976-984, 2016
16.Shitara K, Yonesaka K, Denda T, Yamazaki K, Moriwaki T, Tsuda M, Takano T, Okuda H, Nishina T, Sakai K, Nishio K, Tokunaga S, Yamanaka T, Boku N, Hyodo I, Muro K. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci, 107:1843-1850, 2016
17.Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, Hara H, Ura T, Kojima T, Chin K, Hironaka S, Kii T, Kojima Y, Akutsu Y, Matsushita H, Kawakami K, Mori K, Nagai Y, Asami C, Kitagawa Y. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer, 115:1328-1334, 2016
18.Shitara K, Ohtsu A. Advances in Systemic Therapy for Metastatic or Advanced Gastric Cancer. J Natl Compr Canc Netw, 14:1313-1320, 2016
19.Kasi PM, Kotani D, Cecchini M, Shitara K, Ohtsu A, Ramanathan RK, Hochster HS, Grothey A, Yoshino T. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer, 16:467, 2016
20.Hatogai K, Kitano S, Fujii S, Kojima T, Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget, 7:47252-47264, 2016
21.Satake H, Tahara M, Mochizuki S, Kato K, Hara H, Yokota T, Kiyota N, Kii T, Chin K, Zenda S, Kojima T, Bando H, Yamazaki T, Iwasa S, Honma Y, Hamauchi S, Tsushima T, Ohtsu A. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol, 78:91-99, 2016
22.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
23.Doi T, Lee K-H, Kim T-M, Ohtsu A, Kim TY, Ikeda M, Yoh K, Gallo Stampino C, Hirohashi T, Suzuki A, Fujii Y, Andrew Williams J, Bang Y-J. A phase I study of the human anti-activin receptor-like kinase 1 antibody PF-03446962 in Asian patients with advanced solid tumors. Cancer Med, 5:1454-1463, 2016
24.Doi T, Shitara K, Kojima T, Yoshino T, Dontabhaktuni A, Rebscher H, Tang S, Cosaert J, Ohtsu A. A phase I study evaluating cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, given every 2 or 3 weeks in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77:1253-1262, 2016
25.Yoh K, Doi T, Ohmatsu H, Kojima T, Takahashi H, Zenke Y, Wacheck V, Enatsu S, Nakamura T, Turner K, Uenaka K. A phase I dose-escalation study of LY2875358, a bivalent MET antibody, given as monotherapy or in combination with erlotinib or gefitinib in Japanese patients with advanced malignancies. Invest New Drugs, 34:584-595, 2016
26.Nagatani Y, Shitara K, Bando H, Kuboki Y, Okamoto W, Kojima T, Yoshino T, Nishida T, Ohtsu A, Doi T. Clinical outcomes of patients with gastrointestinal stromal tumor in phase I clinical trials. BMC Cancer, 16:889, 2016
27.Bando H, Doi T, Muro K, Yasui H, Nishina T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, Sato A, Ohtsu A. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer, 62:46-53, 2016
28.Doi A, Sumiyoshi T, Omori Y, Oyamada Y, Kumano K, Yoshizaki N, Hirayama M, Suzuki Y, Okushiba S, Kogawa T, Doi T, Kondo H. Double Extramedullary Plasmacytoma of the Stomach with a Long-term Endoscopic Follow-up. Intern Med, 55:3585-3590, 2016
29.Hatake K, Doi T, Uetake H, Takahashi Y, Ishihara Y, Shirao K. Bevacizumab safety in Japanese patients with colorectal cancer. Jpn J Clin Oncol, 46:234-240, 2016
30.Nishida T, Doi T. Pazopanib for both GIST and soft-tissue sarcoma. Lancet Oncol, 17:549-550, 2016
31.Yoshino T, Kojima T, Bando H, Yamazaki T, Naito Y, Mukai H, Fuse N, Goto K, Ito Y, Doi T, Ohtsu A. Effect of food on the pharmacokinetics of TAS-102 and its efficacy and safety in patients with advanced solid tumors. Cancer Sci, 107:659-665, 2016
32.Osera S, Fujii S, Ikematsu H, Miyamoto H, Oono Y, Yano T, Ochiai A, Yoshino T, Ohtsu A, Kaneko K. Clinicopathological, endoscopic, and molecular characteristics of the "skirt" - a new entity of lesions at the margin of laterally spreading tumors. Endoscopy, 48:448-455, 2016
33.Hatogai K, Yano T, Kojima T, Onozawa M, Fujii S, Daiko H, Yoda Y, Hombu T, Doi T, Kaneko K, Ohtsu A. Local efficacy and survival outcome of salvage endoscopic therapy for local recurrent lesions after definitive chemoradiotherapy for esophageal cancer. Radiat Oncol, 11:31, 2016
34.Hatogai K, Fujii S, Kojima T, Daiko H, Kadota T, Fujita T, Yoshino T, Doi T, Takiguchi Y, Ohtsu A. Prognostic significance of tumor regression grade for patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. J Surg Oncol, 113:390-396, 2016
35.Hatogai K, Yano T, Kojima T, Onozawa M, Daiko H, Nomura S, Yoda Y, Doi T, Kaneko K, Ohtsu A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc, 83:1130-1139 e1133, 2016
36.Kotani D, Shitara K, Kawazoe A, Fukuoka S, Kuboki Y, Bando H, Okamoto W, Kojima T, Doi T, Ohtsu A, Yoshino T. Safety and Efficacy of Trifluridine/Tipiracil Monotherapy in Clinical Practice for Patients With Metastatic Colorectal Cancer: Experience at a Single Institution. Clin Colorectal Cancer, 15:e109-115, 2016