HOME > Publication & Reports > Annual Report 2016 > Hospital East
Department of Experimental Therapeutics
Toshihiko Doi, Kiyotaka Yoh, Yoichi Naito, Takahiro Kogawa, Hideaki Takahashi, Kenichi Harano, Yasutoshi Kuboki, Shigehiro Koganemaru
Introduction
The National Cancer Center (NCC) - the Exploratory Oncology Research & Clinical Trial Center (EPOC) Phase I Group has been organized to promote the early drug development especially the first in human (FIH) trial since 2012. The phase I group consists of two sub-units (NCCE-Kashiwa & NCC-Tsukiji), which are organized by each hospital. The goal of both/each unit is to perform initial clinical evaluations of promising new anti-cancer compounds emerging from laboratories. Our phase I unit is the largest program in Japan and Asia, and we contribute to the development of new cancer drugs through early phase trials.
In April 2013, the Department of Experimental Therapeutics was launched to strongly promote the EPOC missions as previously described. The members of the Department of Experimental Therapeutics consist of specialists of their oncology fields. Also, we have conducted/contributed to invesigator-initiated trial (IIT) using unapproved drugs and academia new seeds.
Routine activities
This department plays an important role in new anti-cancer drug development in our center as well as in Japan. The top priority is to conduct the FIH trials, while we also perform the phase I trials for solid tumors (i.e., all comers). Recently, we joined a global phase I trial to accelerate new drug development in Japan. Web- and tel- conferences are held with EU and US sites, and we discuss patient enrollment as well as further developmental strategy. Routine web-conferences are also held between Kashiwa and Tsukiji campuses every Friday morning, and we share information about adverse events, patient enrollments, and refer candidates to each other in order to accelerate enrollment. Several IIT-FIH using new class seeds are conducted by each unit and also by unapproved company agents.
Research activities
The elucidation of the proof of concept is essential in the new anti-cancer drug development especially in the early phase, so we conduct several translational researches in collaboration with the adjoining Research Institute. In the Kashiwa campus, the comprehensive genomic analyses, which is known as SCRUM-Japan, is ongoing to facilitate patient enrollment for new molecular targeted drugs under investigation. Also, new immune-monitoring system for immune agents is established in the NCC-Hospital East, which is controlled by Prof. Nishikawa.
Clinical trials
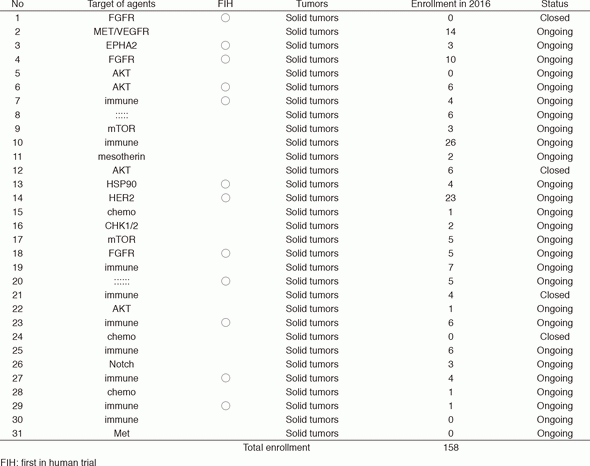
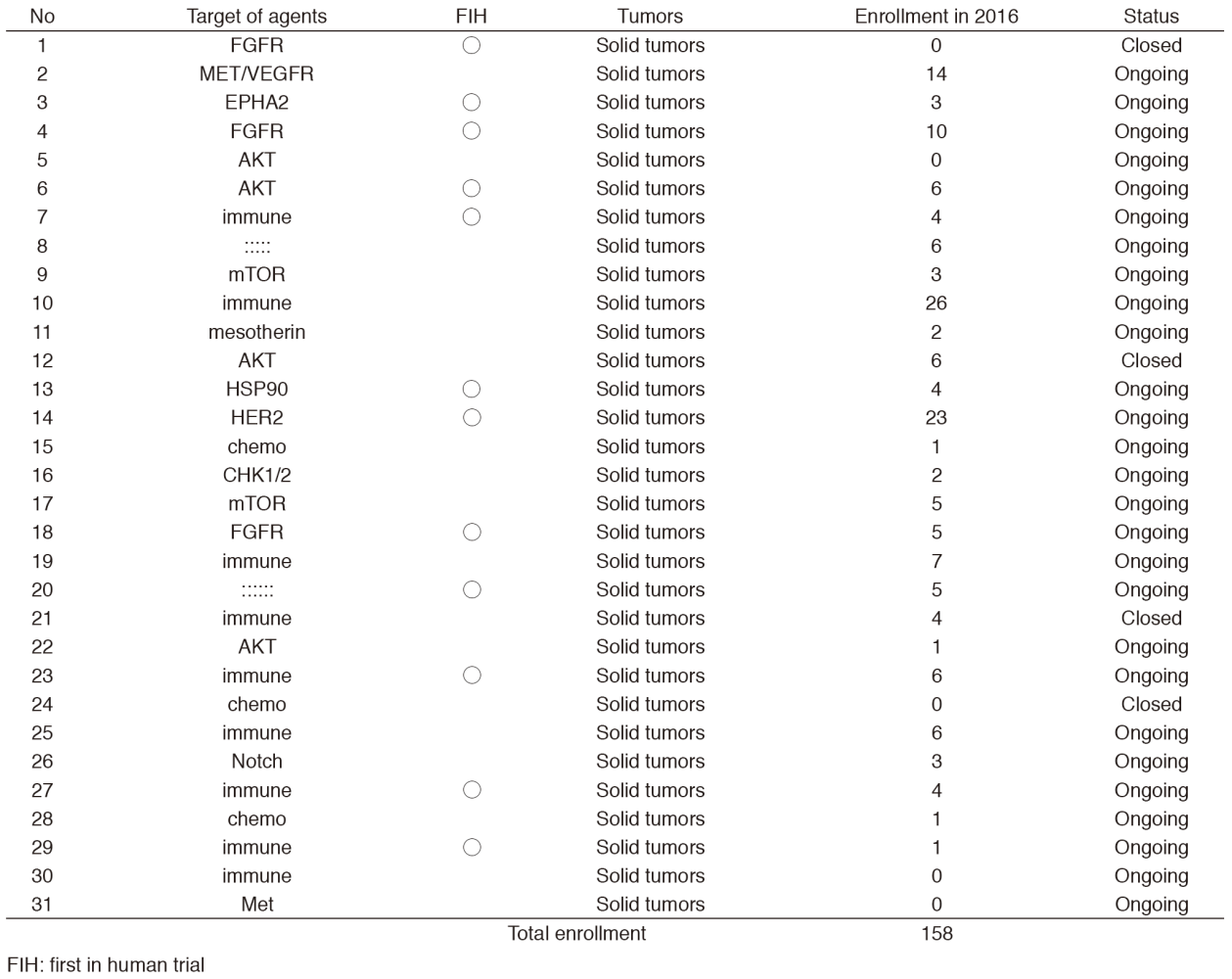
In 2016, 31 phase I trials were conducted (Table 1).
List of papers published in 2016
Journal
1.Shitara K, Muro K, Shimada Y, Hironaka S, Sugimoto N, Komatsu Y, Nishina T, Yamaguchi K, Segawa Y, Omuro Y, Tamura T, Doi T, Yukisawa S, Yasui H, Nagashima F, Gotoh M, Esaki T, Emig M, Chandrawansa K, Liepa AM, Wilke H, Ichimiya Y, Ohtsu A. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer, 19:927-938, 2016
2.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Okuyama H, Ueno H, Morizane C, Kondo S, Sakamoto Y, Okusaka T, Ochiai A. C-Reactive Protein Level Is an Indicator of the Aggressiveness of Advanced Pancreatic Cancer. Pancreas, 45:110-116, 2016
3.Harano K, Yonemori K, Hirakawa A, Shimizu C, Katsumata N, Gemma A, Fujiwara Y, Tamura K. The influence of familial factors on the choice of the place of death for terminally ill breast cancer patients: a retrospective single-center study. Breast Cancer, 23:797-806, 2016
4.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Prognostic factors in patients with uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Int J Clin Oncol, 21:168-176, 2016
5.Kuboki Y, Yamashita S, Niwa T, Ushijima T, Nagatsuma A, Kuwata T, Yoshino T, Doi T, Ochiai A, Ohtsu A. Comprehensive analyses using next-generation sequencing and immunohistochemistry enable precise treatment in advanced gastric cancer. Ann Oncol, 27:127-133, 2016
6.Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer, 19:183-191, 2016
7.Ikeda M, Sato A, Mochizuki N, Toyosaki K, Miyoshi C, Fujioka R, Mitsunaga S, Ohno I, Hashimoto Y, Takahashi H, Hasegawa H, Nomura S, Takahashi R, Yomoda S, Tsuchihara K, Kishino S, Esumi H. Phase I trial of GBS-01 for advanced pancreatic cancer refractory to gemcitabine. Cancer Sci, 107:1818-1824, 2016
8.Ikeda M, Yamamoto H, Kaneko M, Oshima H, Takahashi H, Umemoto K, Watanabe K, Hashimoto Y, Ohno I, Mitsunaga S, Okusaka T. Screening rate for hepatitis B virus infection in patients undergoing chemotherapy in Japan. Int J Clin Oncol, 21:1162-1166, 2016
9.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Optimal cytoreductive surgery in patients with advanced uterine carcinosarcoma: A multi-institutional retrospective study from the Japanese gynecologic oncology group. Gynecol Oncol, 141:447-453, 2016
10.Hatogai K, Kitano S, Fujii S, Kojima T, Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget, 7:47252-47264, 2016
11.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
12.Harano K, Lei X, Gonzalez-Angulo AM, Murthy RK, Valero V, Mittendorf EA, Ueno NT, Hortobagyi GN, Chavez-MacGregor M. Clinicopathological and surgical factors associated with long-term survival in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat, 159:367-374, 2016
13.Doi T, Lee K-H, Kim T-M, Ohtsu A, Kim TY, Ikeda M, Yoh K, Gallo Stampino C, Hirohashi T, Suzuki A, Fujii Y, Andrew Williams J, Bang Y-J. A phase I study of the human anti-activin receptor-like kinase 1 antibody PF-03446962 in Asian patients with advanced solid tumors. Cancer Med, 5:1454-1463, 2016
14.Doi T, Shitara K, Kojima T, Yoshino T, Dontabhaktuni A, Rebscher H, Tang S, Cosaert J, Ohtsu A. A phase I study evaluating cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, given every 2 or 3 weeks in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77:1253-1262, 2016
15.Yoh K, Doi T, Ohmatsu H, Kojima T, Takahashi H, Zenke Y, Wacheck V, Enatsu S, Nakamura T, Turner K, Uenaka K. A phase I dose-escalation study of LY2875358, a bivalent MET antibody, given as monotherapy or in combination with erlotinib or gefitinib in Japanese patients with advanced malignancies. Invest New Drugs, 34:584-595, 2016
16.Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, Enatsu S, Lee P, Ferry D, Tamura T, Nakagawa K. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer, 99:186-193, 2016
17.Kogawa T, Fouad TM, Liu DD, Wu J, Shen Y, Masuda H, Fujii T, Chavez-MacGregor M, Alvarez RH, Hortobagyi GN, Valero V, Ueno NT. High HER2/Centromeric Probe for Chromosome 17 Fluorescence In Situ Hybridization Ratio Predicts Pathologic Complete Response and Survival Outcome in Patients Receiving Neoadjuvant Systemic Therapy With Trastuzumab for HER2-Overexpressing Locally Advanced Breast Cancer. Oncologist, 21:21-27, 2016
18.Nagatani Y, Shitara K, Bando H, Kuboki Y, Okamoto W, Kojima T, Yoshino T, Nishida T, Ohtsu A, Doi T. Clinical outcomes of patients with gastrointestinal stromal tumor in phase I clinical trials. BMC Cancer, 16:889, 2016
19.Bando H, Doi T, Muro K, Yasui H, Nishina T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, Sato A, Ohtsu A. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer, 62:46-53, 2016
20.Doi A, Sumiyoshi T, Omori Y, Oyamada Y, Kumano K, Yoshizaki N, Hirayama M, Suzuki Y, Okushiba S, Kogawa T, Doi T, Kondo H. Double Extramedullary Plasmacytoma of the Stomach with a Long-term Endoscopic Follow-up. Intern Med, 55:3585-3590, 2016
21.Hatake K, Doi T, Uetake H, Takahashi Y, Ishihara Y, Shirao K. Bevacizumab safety in Japanese patients with colorectal cancer. Jpn J Clin Oncol, 46:234-240, 2016
22.Nishida T, Doi T. Pazopanib for both GIST and soft-tissue sarcoma. Lancet Oncol, 17:549-550, 2016
23.Yoshino T, Kojima T, Bando H, Yamazaki T, Naito Y, Mukai H, Fuse N, Goto K, Ito Y, Doi T, Ohtsu A. Effect of food on the pharmacokinetics of TAS-102 and its efficacy and safety in patients with advanced solid tumors. Cancer Sci, 107:659-665, 2016
24.Saruwatari K, Umemura S, Nomura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ohe Y, Goto K. Prognostic Factor Analysis in Patients With Small-Cell Lung Cancer Treated With Third-Line Chemotherapy. Clin Lung Cancer, 17:581-587, 2016
25.Zenke Y, Umemura S, Sugiyama E, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Goto K. Successful treatment with afatinib after grade 3 hepatotoxicity induced by both gefitinib and erlotinib in EGFR mutation-positive non-small cell lung cancer. Lung Cancer, 99:1-3, 2016
26.Zenke Y, Umemura S, Motegi A, Furukawa K, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Tsuboi M, Akimoto T, Goto K. Acute and Progressive Tracheal Stenosis after Proton Beam Therapy with Concurrent Chemotherapy for Non-Small Cell Lung Cancer. J Thorac Oncol, 11:1181-1183, 2016
27.Zenke Y, Yoh K, Matsumoto S, Umemura S, Niho S, Ohmatsu H, Goto K, Ohe Y. Clinical Impact of Gastric Acid-Suppressing Medication Use on the Efficacy of Erlotinib and Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer Harboring EGFR Mutations. Clin Lung Cancer, 17:412-418, 2016
28.Tamiya M, Tamiya A, Kaneda H, Nakagawa K, Yoh K, Goto K, Okamoto H, Shimokawa T, Abe T, Tanaka H, Daga H, Takeda K, Hirashima T, Atagi S. A phase II study of pemetrexed plus carboplatin followed by maintenance pemetrexed as first-line chemotherapy for elderly patients with advanced non-squamous non-small cell lung cancer. Med Oncol, 33:2, 2016
29.Mukai H, Saeki T, Aogi K, Naito Y, Matsubara N, Shigekawa T, Ueda S, Takashima S, Hara F, Yamashita T, Ohwada S, Sasaki Y. Patritumab plus trastuzumab and paclitaxel in human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Cancer Sci, 107:1465-1470, 2016
30.Hisakane K, Saruwatari K, Fujii S, Kirita K, Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, Ochiai A, Gemma A, Tsuboi M, Goto K, Ishii G. Unique intravascular tumor microenvironment predicting recurrence of lung squamous cell carcinoma. J Cancer Res Clin Oncol, 142:593-600, 2016
31.Saruwatari K, Ikemura S, Sekihara K, Kuwata T, Fujii S, Umemura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ochiai A, Kohrogi H, Tsuboi M, Goto K, Ishii G. Aggressive tumor microenvironment of solid predominant lung adenocarcinoma subtype harboring with epidermal growth factor receptor mutations. Lung Cancer, 91:7-14, 2016
32.Matsuzawa R, Kirita K, Kuwata T, Umemura S, Matsumoto S, Fujii S, Yoh K, Kojima M, Niho S, Ohmatsu H, Ochiai A, Tsuboi M, Goto K, Ishii G. Factors influencing the concordance of histological subtype diagnosis from biopsy and resected specimens of lung adenocarcinoma. Lung Cancer, 94:1-6, 2016
33.Hatogai K, Yano T, Kojima T, Onozawa M, Fujii S, Daiko H, Yoda Y, Hombu T, Doi T, Kaneko K, Ohtsu A. Local efficacy and survival outcome of salvage endoscopic therapy for local recurrent lesions after definitive chemoradiotherapy for esophageal cancer. Radiat Oncol, 11:31, 2016
34.Hatogai K, Fujii S, Kojima T, Daiko H, Kadota T, Fujita T, Yoshino T, Doi T, Takiguchi Y, Ohtsu A. Prognostic significance of tumor regression grade for patients with esophageal squamous cell carcinoma after neoadjuvant chemotherapy followed by surgery. J Surg Oncol, 113:390-396, 2016
35.Hatogai K, Yano T, Kojima T, Onozawa M, Daiko H, Nomura S, Yoda Y, Doi T, Kaneko K, Ohtsu A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc, 83:1130-1139 e1133, 2016
36.Kotani D, Shitara K, Kawazoe A, Fukuoka S, Kuboki Y, Bando H, Okamoto W, Kojima T, Doi T, Ohtsu A, Yoshino T. Safety and Efficacy of Trifluridine/Tipiracil Monotherapy in Clinical Practice for Patients With Metastatic Colorectal Cancer: Experience at a Single Institution. Clin Colorectal Cancer, 15:e109-115, 2016