HOME > Publication & Reports > Annual Report 2016 > Hospital East
Clinical Research Support Office
Akihiro Sato
- Research Management Section
Miki Fukutani, Sakiko Kuroda, Takako Tomizawa, Kayo Toyosaki, Masako Nakamoto, Kana Fukui, Hitomi Tamura, Masato Yonemura, Noriko Fujishiro - Data Management Section
Hiromi Hasegawa, Yoshihiro Aoyagi, Seiko Matshuda, Kaori Tobayama, Ayako Sugama, Tshukiko Higuchi - Bio Bank and Translational Research Support Section
Wataru Okamoto, Akiko Nakayama, Izumi Miki, Tomohisa Sudo, Yuuko Tagami - Clinical Research Coordinating Section
Takayuki Yoshino, Masafumi Ikeda, Yoichi Naito, Kiyotaka Yoh, Takashi Kojima, Nobuaki Matsubara, Yasutoshi Kuboki, Takahiro Sakai, Yukie Kimura, Miyuki Hara, Yuko Ito, Ikumi Tamaki, Mayumi Shirase, Keiko Yamamoto, Chiyo Ito, Koko Komata, Aya Shinbu, Chiharu Hirano, Kiyoko Adachi, Keiko Abe, Rie Taniguchi, Emi Numata, Kyoko Uehara, Noriko Noda, Yoshimi Fujiki, Junko Tsukada, Megumi Hodota, Rikako Tanaka, Megumi Futamura, Masami Sasaki, Mizuho Ibaraki, Aki Hashimoto, Mikina Takiguchi, Tomoko Matsumura, Atsuko Katagiri, Haruna Ariyoshi, Hikaru Matsuki, Yasuko Nishikubo, Masumi Kudo, Yoshimi Izumi, Minako Suzuki, Mayumi Nagino, Fumiko Kase, Megumi Nakanishi, Tomoko Watanabe
Introduction
The Research Management Section and the Data Management Section support the investigator initiated clinical trial program conducted in the National Cancer Center Hospital East (NCCHE) through the clinical datacenter, study management, site visit monitoring, safety information management, and bio statistics.
The Bio Bank and Translational Research Support Section supports Translational Research (TR) ex. Genome wide screening network program through study management and data management.
Routine activities
1.Data Management Section
- Data base and CRF (Case Report Form) design
- Registration
- Data management
- System administration
- Bio statistics
2.Research Management Section
- Study management
- Site visit monitoring
- Safety information management
- Medical writing
3.Bio Bank and Translational Research Support Section
- Study management for TR
- Data management for TR
- Research concierges for bio bank
4.Clinical Research Coordinating Section
- Clinical Research Coordination
Clinical trials
1.Data Management and Research Management Sections
- In 2016, three IND (investigational new drug) trials were started their enrollment.
- A total of 67 patients was enrolled.
2.Bio Bank and Translational Research Support Section
- In 2016, five TR trials were conducted.
- A total of 1,045 patients was enrolled.
3.Clinical Research Coordinating Section
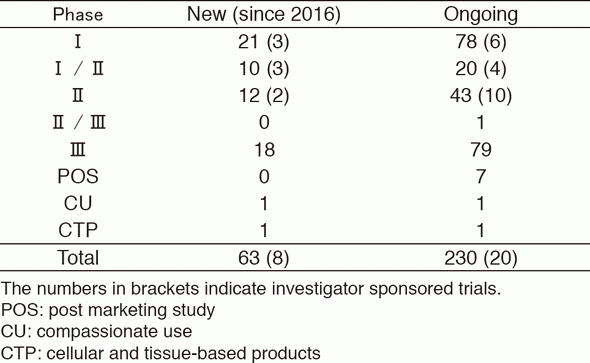
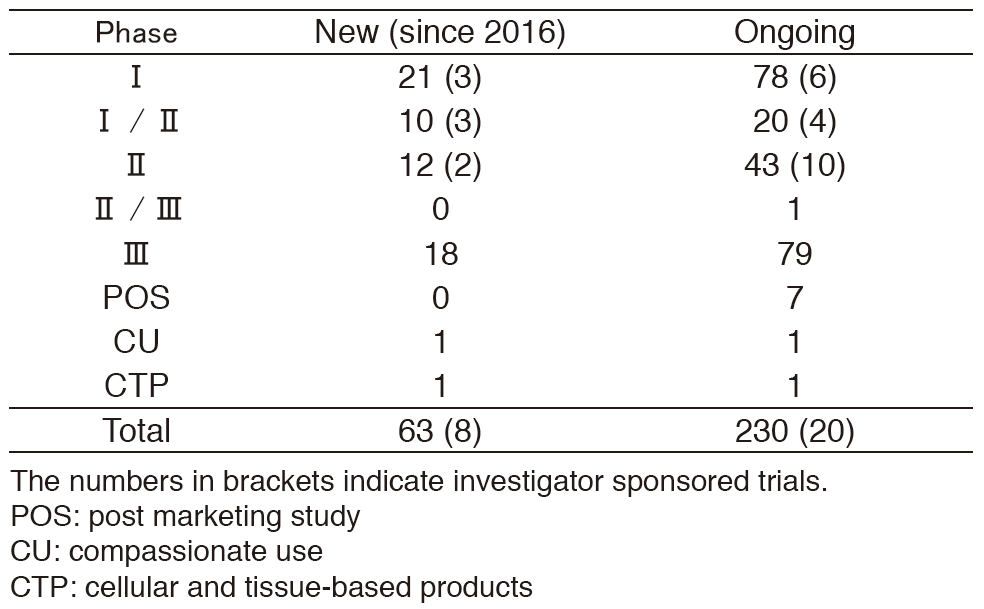
-As shown on Table 1.
Table 1. Supported company or investigator sponsored trials in the Clinical Research Coordinating Section in 2016
Education
-On-the-job training for new staff
-Support the education programs for clinical trial methodology and GCP (good clinical practice) in the NCC.
-Co-host the GCP training seminar with other AROs (academic research organizations).
Future prospects
-Preparation for conductiing global IIT (investigator initiated trial) IND trials
List of papers published in 2016
Journal
1.Ikeda M, Sato A, Mochizuki N, Toyosaki K, Miyoshi C, Fujioka R, Mitsunaga S, Ohno I, Hashimoto Y, Takahashi H, Hasegawa H, Nomura S, Takahashi R, Yomoda S, Tsuchihara K, Kishino S, Esumi H. Phase I trial of GBS-01 for advanced pancreatic cancer refractory to gemcitabine. Cancer Sci, 107:1818-1824, 2016
2.Bando H, Doi T, Muro K, Yasui H, Nishina T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, Sato A, Ohtsu A. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer, 62:46-53, 2016
