HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Breast and Medical Oncology
Kenji Tamura, Chikako Shimizu, Kan Yonemori, Mayu Yunokawa, Emi Noguchi, Tatsunori Shimoi, Akihiko Shimomura, Atsuko Kitano, Tadaaki Nishikawa, Hitomi Okuma, Asuka Kawachi, Yasuhiro Fujiwara
Introduction
The Department of Breast and Medical Oncology provides the most effective treatment by the use of chemotherapy, and works on the establishment of new standard care for adult malignancies including breast cancer, gynecologic cancer, soft-tissue sarcoma, extragonadal germ cell tumors, primary unknown tumors, and other rare types of solid tumors.
We envision becoming a premier medical oncology department, which leads cancer care in Japan and in the world. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatment through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented, and multi-disciplinary approach is the core value of our practice.
Our team and what we do
1.Setup
Our department consists of eight full-time attending physicians, four chief residents (fellows), and two to three clinical residents. We also provide educational opportunities to short-term (a half year) residents. Full-time attending physicians are on duty at the outpatient clinic two to three days per week. The management of hospitalized patients is undertaken by clinical teams consisting of attending physicians and residents. A Grand Round is scheduled every Wednesday and Friday.
2.Performance
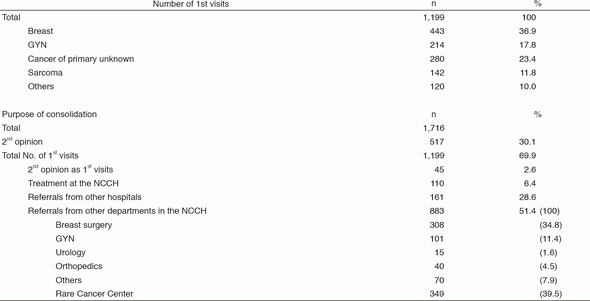
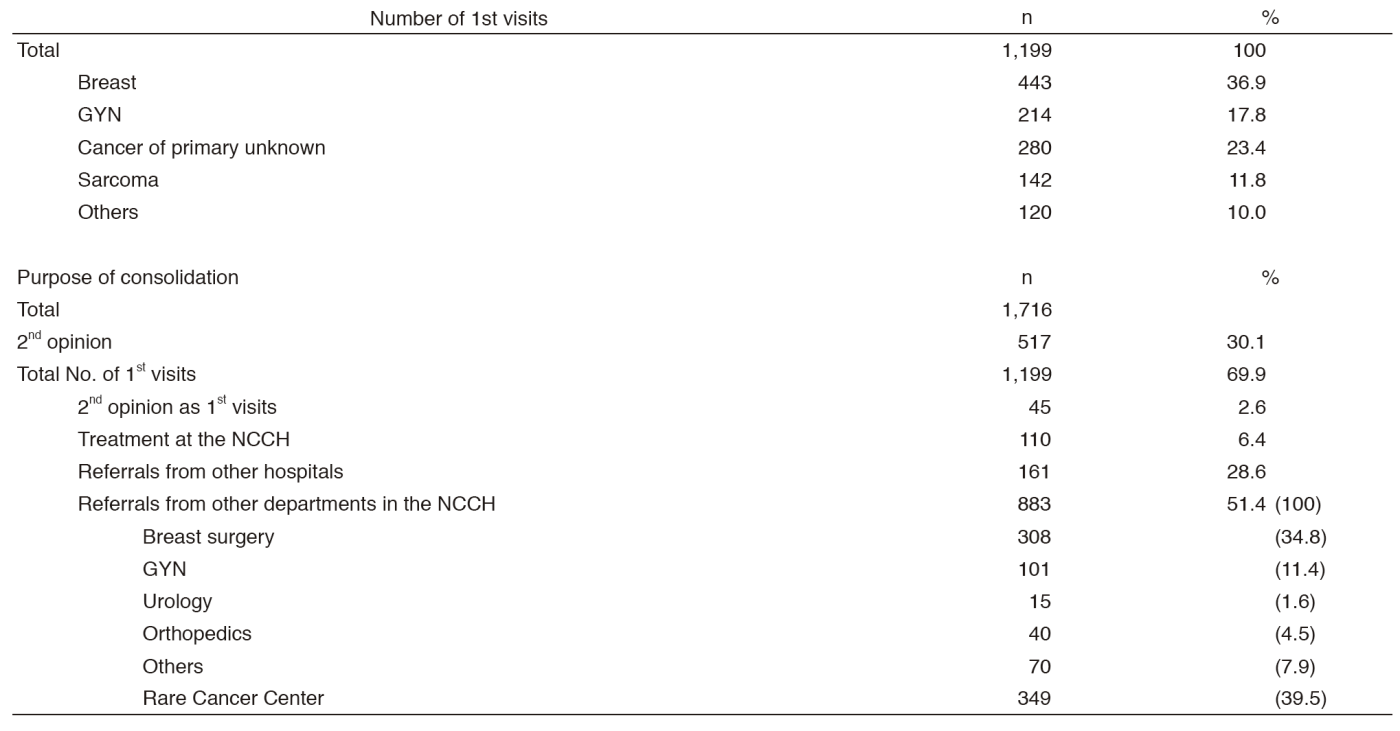
There were 1,199 first visits of new patients and 517 for second opinions in 2016 (Table 1). Thirty-seven percent of new patients are breast cancer patients, 18% are gynecological cancer patients, 23% are patients with cancer of primary unknown, and 12% are patients with soft-tissue sarcoma. Approximately 51% of new patients were referred from other departments of the NCCH, and 29% from other hospitals. Approximately 35% of referrals from the NCCH were from the Department (Dept.) of Breast Surgery, 11% from the Dept. of Gynecology, 1.6% from Urology, 4.5% from Musculoskeletal Oncology and Rehabilitation, and 40% from the Rare Cancer Center. We have approximately 36.3 inpatients daily. We care 1,942 outpatients per month; 109 outpatients per day (ranked first among all departments). The number of outpatient who received chemotherapy delivered by our department was 13,452 per year, which accounts for 36.7% of the total number and ranks first in the number of treatments delivered at the Outpatient Treatment Center.
3.Conference
A one-hour briefing medical conference is held every morning to discuss the evidence-based care for individual patients. A conference on Phase I is held on Monday, Journal Club on Wednesday, Clinical trials conference on Thursday, and the Weekend and Outpatient follow-up conference on Friday. Multidisciplinary Case Conferences with diagnostic radiologists, surgeons, and pathologists are held with members of the Departments of Breast Surgery, Gynecology, Musculoskeletal Oncology and Rehabilitation, Radiation Oncology, and the Pathology Division once or twice (Breast) per week, respectively.
The Monthly Breast Cancer Conference is held with the participation of multidisciplinary specialists to discuss recent topics in breast oncology and to update institutional treatment guidelines. In 2014, we published the "Nyugan-shinryou Application Note" from Nanzando based on this guideline, which reflects the consensus of the breast team on the body of evidence on breast cancer management.
Research activities
Our research interest extends across a wide range of topics related to treatment and clinical program development. A lot of our researches are secured by public and consignment research grants. In 2016, we conducted many research programs as the primary investigator and participated in additional programs as the co-investigator secured by competitive public research funds. We published 32 international manuscripts, focusing on early phase anti-cancer drug development, molecular imaging, translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarker to predict efficacy and adverse event of anti-cancer drugs, and other basic research. We value cancer survivorship as a research theme in order to develop a patient-centered comprehensive care program.
Clinical trials
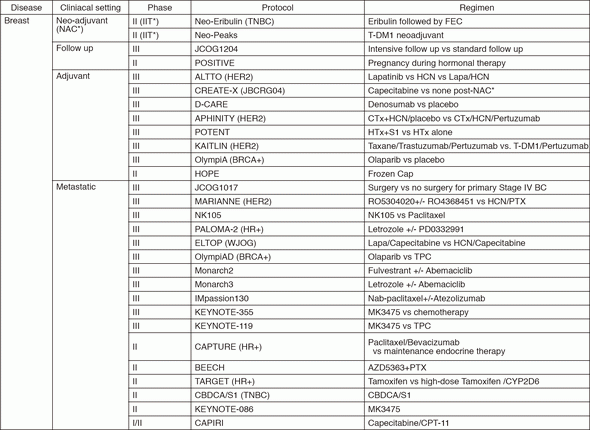
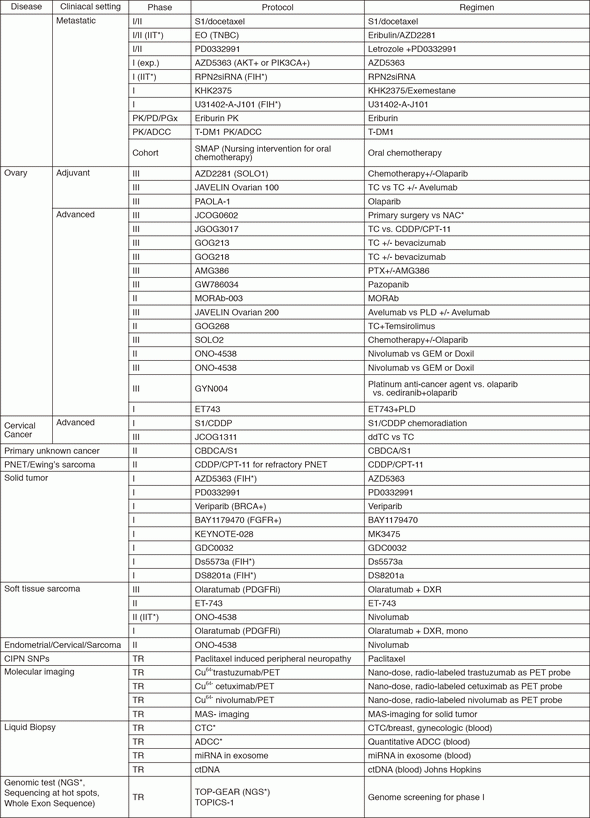
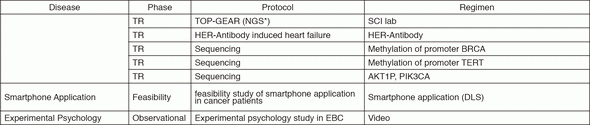
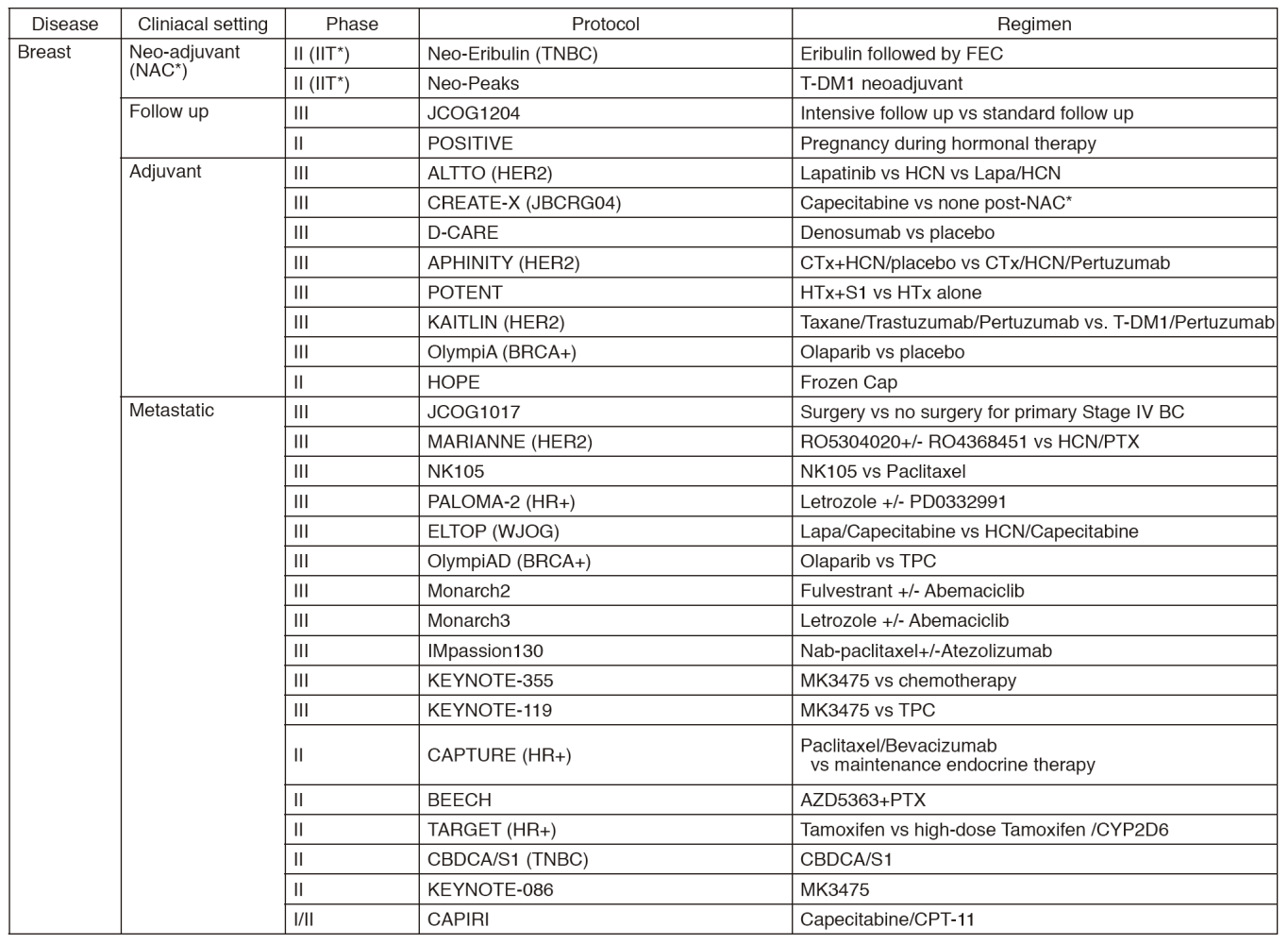
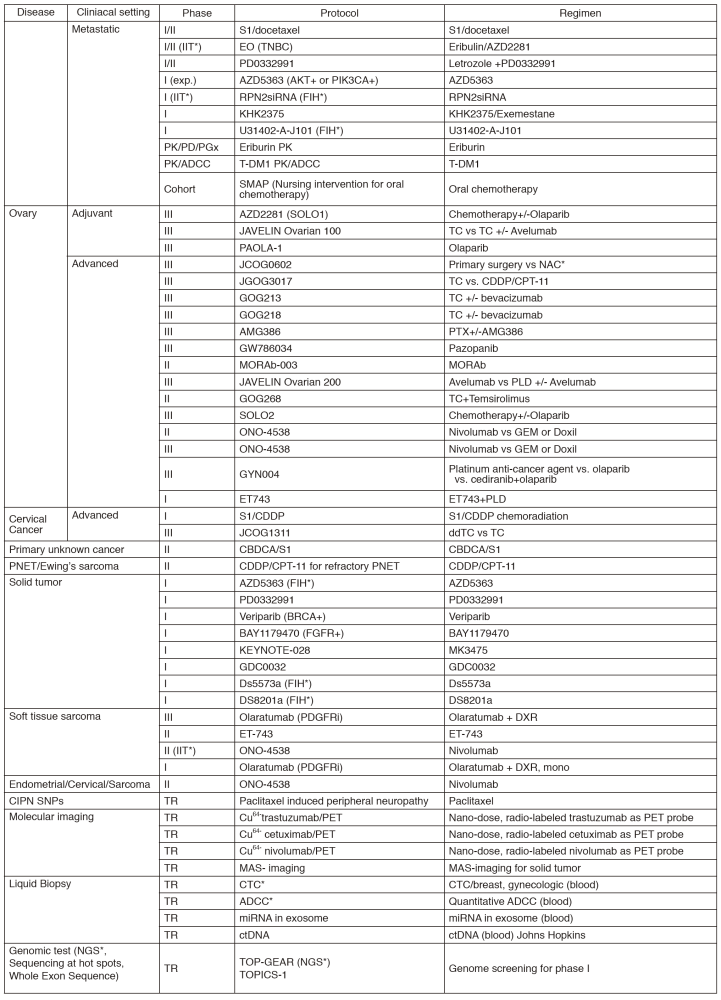
In 2016, we actively enrolled patients in phase I studies (including first in human or global) as well as national and international phase II and III studies (Table 2). Of note, we launched a pharmacokinetic and dose-finding study of eribulin/olaparib, a phase II study of eribulin in neoadjuvant setting in triple negative breast cancer, T-DM1 neoadjuvant, ONO-4538, and phase I of RPN2 (first in human) as an investigator-initiated clinical trial (IIT in Table 2). New molecular imaging studies are launched in cooperation with research institutes. We also conducted many types of prospective cohort translational studies to find novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experiences as well as research activities. Residents are encouraged to make presentations at local and national conferences. We vigorously support basic, clinical, or translational researches conducted by postgraduate students.
Future prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes a tumor registry, translational research, clinical trials, and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 1. 1st visiting patients to the Department of Breast and Medical Oncology (January - December 2016)
List of papers published in 2016
Journal
1.Harano K, Yonemori K, Hirakawa A, Shimizu C, Katsumata N, Gemma A, Fujiwara Y, Tamura K. The influence of familial factors on the choice of the place of death for terminally ill breast cancer patients: a retrospective single-center study. Breast Cancer, 23:797-806, 2016
2.Kurihara H, Shimizu C, Miyakita Y, Yoshida M, Hamada A, Kanayama Y, Yonemori K, Hashimoto J, Tani H, Kodaira M, Yunokawa M, Yamamoto H, Watanabe Y, Fujiwara Y, Tamura K. Molecular imaging using PET for breast cancer. Breast Cancer, 23:24-32, 2016
3.Shimizu C, Mogushi K, Morioka MS, Yamamoto H, Tamura K, Fujiwara Y, Tanaka H, . Fc-Gamma receptor polymorphism and gene expression of peripheral blood mononuclear cells in patients with HER2-positive metastatic breast cancer receiving single-agent trastuzumab. Breast Cancer, 23:624-632, 2016
4.Sawada T, Watanabe M, Fujimura Y, Yagishita S, Shimoyama T, Maeda Y, Kanda S, Yunokawa M, Tamura K, Tamura T, Minami H, Koh Y, Koizumi F. Sensitive cytometry based system for enumeration, capture and analysis of gene mutations of circulating tumor cells. Cancer Sci, 107:307-314, 2016
5.Shimomura A, Fujiwara Y, Kondo S, Kodaira M, Iwasa S, Kitano S, Tanabe Y, Tamura K, Yamamoto N. Tremelimumab-associated tumor regression following after initial progression: two case reports. Immunotherapy, 8:9-15, 2016
6.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Prognostic factors in patients with uterine carcinosarcoma: a multi-institutional retrospective study from the Japanese Gynecologic Oncology Group. Int J Clin Oncol, 21:168-176, 2016
7.Yamashita M, Kitano S, Aikawa H, Kuchiba A, Hayashi M, Yamamoto N, Tamura K, Hamada A. A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells. Sci Rep, 6:19772, 2016
8.Okuma HS, Kobayashi Y, Makita S, Kitahara H, Fukuhara S, Munakata W, Suzuki T, Maruyama D, Tobinai K. Disseminated herpes zoster infection initially presenting with abdominal pain in patients with lymphoma undergoing conventional chemotherapy: A report of three cases. Oncol Lett, 12:809-814, 2016
9.Yamagishi M, Tsuta K, Shimoi T, Tanabe Y, Hirai M, Kohno T, Shiraishi K, Nakaoku T, Sunami K, Shimada Y, Tamura K, Hamada A. Automated and rapid system for detection of ALK rearrangement genes in non-small cell lung cancer based on a Quenching Probe method. Biomed Res Clin Prac, 1:36-41, 2016
10.Mukai H, Arihiro K, Shimizu C, Masuda N, Miyagi Y, Yamaguchi T, Yoshida T. Stratifying the outcome after neoadjuvant treatment using pathological response classification by the Japanese Breast Cancer Society. Breast Cancer, 23:73-77, 2016
11.Fujiwara Y, . Evolution of frameworks for expediting access to new drugs in Japan. Nat Rev Drug Discov, 15:293-294, 2016
12.Stopeck AT, Fizazi K, Body J-J, Brown JE, Carducci M, Diel I, Fujiwara Y, Martin M, Paterson A, Tonkin K, Shore N, Sieber P, Kueppers F, Karsh L, Yardley D, Wang H, Maniar T, Arellano J, Braun A, . Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer. Support Care Cancer, 24:447-455, 2016
13.Miyoshi Y, Yorifuji T, Horikawa R, Takahashi I, Nagasaki K, Ishiguro H, Fujiwara I, Ito J, Oba M, Kawamoto H, Fujisaki H, Kato M, Shimizu C, Kato T, Matsumoto K, Sago H, Takimoto T, Okada H, Suzuki N, Yokoya S, Ogata T, Ozono K. Gonadal function, fertility, and reproductive medicine in childhood and adolescent cancer patients: a national survey of Japanese pediatric endocrinologists. Clin Pediatr Endocrinol, 25:45-57, 2016
14.Bun S, Yunokawa M, Ebata T, Shimomura A, Shimoi T, Kodaira M, Yonemori K, Shimizu C, Fujiwara Y, Kato T, Makino Y, Hayashi Y, Tamura K. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother Pharmacol, 78:745-752, 2016
15.Ebata T, Yunokawa M, Bun S, Shimomura A, Shimoi T, Kodaira M, Yonemori K, Shimizu C, Fujiwara Y, Kato T, Tamura K. Dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube, or primary peritoneal carcinomas. Cancer Chemother Pharmacol, 78:1283-1288, 2016
16.Fujiwara K, Shintani D, Nishikawa T. Clear-cell carcinoma of the ovary. Ann Oncol, 27 Suppl 1:i50-i52, 2016
17.Fujiwara Y, Tamura K, Kondo S, Tanabe Y, Iwasa S, Shimomura A, Kitano S, Ogasawara K, Turner PK, Mori J, Asou H, Chan EM, Yamamoto N. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol, 78:281-288, 2016
18.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Optimal cytoreductive surgery in patients with advanced uterine carcinosarcoma: A multi-institutional retrospective study from the Japanese gynecologic oncology group. Gynecol Oncol, 141:447-453, 2016
19.Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, Sheridan TB, Bush SH, Klobocista MM, Blake EA, Takano T, Matsuzaki S, Baba T, Satoh S, Shida M, Nishikawa T, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Fujiwara K, Hazama Y, Kadogami D, Moffitt MN, Takeuchi S, Nishimura M, Iwasaki K, Ushioda N, Johnson MS, Yoshida M, Hakam A, Li SW, Richmond AM, Machida H, Mhawech-Fauceglia P, Ueda Y, Yoshino K, Yamaguchi K, Oishi T, Kajiwara H, Hasegawa K, Yasuda M, Kawana K, Suda K, Miyake TM, Moriya T, Yuba Y, Morgan T, Fukagawa T, Wakatsuki A, Sugiyama T, Pejovic T, Nagano T, Shimoya K, Andoh M, Shiki Y, Enomoto T, Sasaki T, Mikami M, Shimada M, Konishi I, Kimura T, Post MD, Shahzad MM, Im DD, Yoshida H, Omatsu K, Ueland FR, Kelley JL, Karabakhtsian RG, Roman LD. Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann Oncol, 27:1257-1266, 2016
20.Miyasaka A, Nishikawa T, Kozawa E, Yasuda M, Fujiwara K, Hasegawa K. Advanced Mucinous Adenocarcinoma Arising from a Mature Cystic Teratoma: A Case Report and Literature Review. Case Rep Oncol, 9:331-337, 2016
21.Nagao S, Iwasa N, Kurosaki A, Nishikawa T, Hanaoka T, Hasegawa K, Fujiwara K. The Efficacy of Low-Dose Paclitaxel Added to Combination Chemotherapy of Carboplatin and Gemcitabine or Pegylated Liposomal Doxorubicin. Int J Gynecol Cancer, 26:443-448, 2016
22.Okuma HS, Koizumi F, Hirakawa A, Nakatochi M, Komori O, Hashimoto J, Kodaira M, Yunokawa M, Yamamoto H, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Clinical and microarray analysis of breast cancers of all subtypes from two prospective preoperative chemotherapy studies. Br J Cancer, 115:411-419, 2016
23.Okuma HS, Kondo S. Trends in the development of MET inhibitors for hepatocellular carcinoma. Future Oncol, 12:1275-1286, 2016
24.Sasada S, Kodaira M, Shimoi T, Shimomura A, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Ifosfamide and Etoposide Chemotherapy in the Treatment of Recurrent/Refractory Rhabdomyosarcoma in Adults. Anticancer Res, 36:2429-2432, 2016
25.Tamura K, Hashimoto J, Tanabe Y, Kodaira M, Yonemori K, Seto T, Hirai F, Arita S, Toyokawa G, Chen L, Yamamoto H, Kawata T, Lindemann J, Esaki T. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77:787-795, 2016
26.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
27.Tanabe Y, Ichikawa H, Kohno T, Yoshida H, Kubo T, Kato M, Iwasa S, Ochiai A, Yamamoto N, Fujiwara Y, Tamura K. Comprehensive screening of target molecules by next-generation sequencing in patients with malignant solid tumors: guiding entry into phase I clinical trials. Mol Cancer, 15:73, 2016
28.Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Takahashi N, Hashimoto J, Kodaira M, Yamamoto H, Yunokawa M, Shimizu C, Fujimoto M, Fujiwara Y, Tamura K. Risk Factors for Developing Skeletal-Related Events in Breast Cancer Patients With Bone Metastases Undergoing Treatment With Bone-Modifying Agents. Oncologist, 21:508-513, 2016
29.Sawada T, Araki J, Yamashita T, Masubuchi M, Chiyoda T, Yunokawa M, Hoshi K, Tao S, Yamamura S, Yatsushiro S, Abe K, Kataoka M, Shimoyama T, Maeda Y, Kuroi K, Tamura K, Sawazumi T, Minami H, Suda Y, Koizumi F. Prognostic Impact of Circulating Tumor Cell Detected Using a Novel Fluidic Cell Microarray Chip System in Patients with Breast Cancer. EBioMedicine, 11:173-182, 2016
30.Yonemori K, Hirakawa A, Kawachi A, Kinoshita F, Okuma H, Nishikawa T, Tamura K, Fujiwara Y, Takebe N. Drug induced interstitial lung disease in oncology phase I trials. Cancer Sci, 107:1830-1836, 2016
31.Yonemori K, Tamura K, Kodaira M, Fujikawa K, Sagawa T, Esaki T, Shirakawa T, Hirai F, Yokoi Y, Kawata T, Hatano B, Takahashi Y. Safety and tolerability of the olaparib tablet formulation in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol, 78:525-531, 2016
32.Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S, Shimizu C, Fujiwara Y, Kinoshita T, Tamura K, Ochiya T. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci, 107:326-334, 2016