HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Thoracic Oncology
Yuichiro Ohe, Noboru Yamamoto, Hiroshi Nokihara, Yutaka Fujiwara, Hidehito Horinouchi, Shintaro Kanda, Yasushi Goto, Kota Itahashi, Hideaki Shiraishi, Keiko Goto
Introduction
Lung cancer is the leading cause of cancer death in Japan and worldwide. The incidence of lung cancer is still increasing in Japan, especially in elderly populations. The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The goals of our department are to provide the highest quality treatment and establish new effective treatments for lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, staff members of our department work closely with thoracic surgeons, radiation oncologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Our department includes seven staff physicians. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
Our team and what we do
The staff physicians attend outpatient services for thoracic diseases, and our department has approximately 60 beds in the hospital. Inpatient care is carried out by five teams. Each team consists of one staff physician and one or two residents and/or trainee doctors. Protocol and case conferences are scheduled every Monday morning and afternoon, respectively. The journal club is scheduled on Thursday mornings.
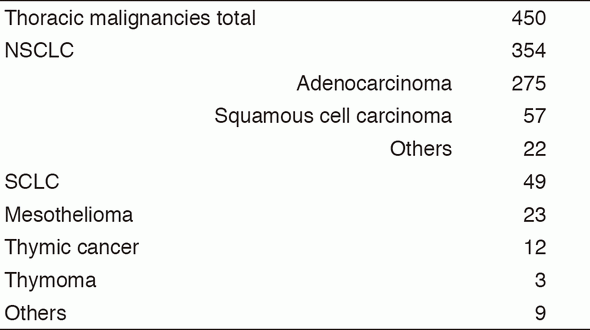
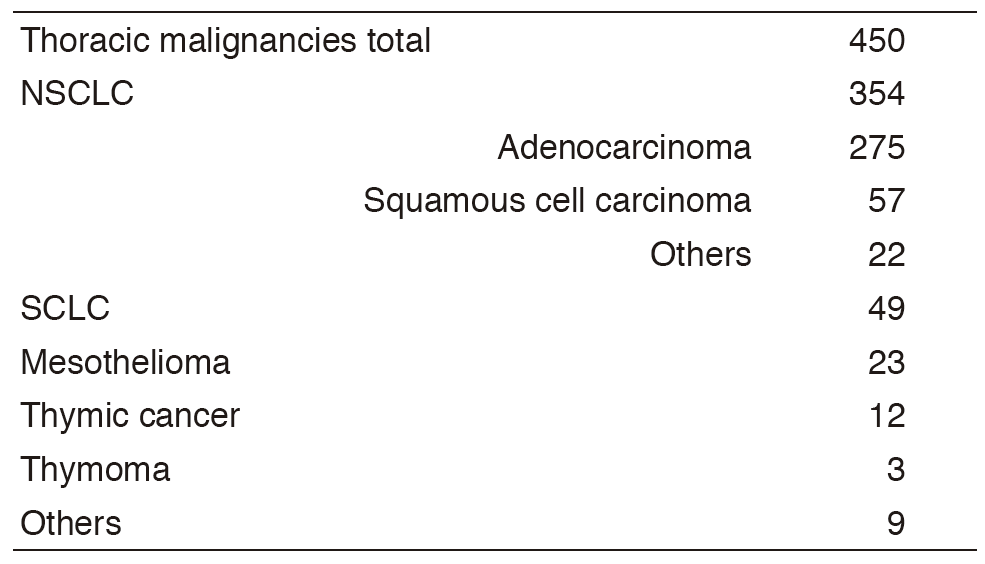
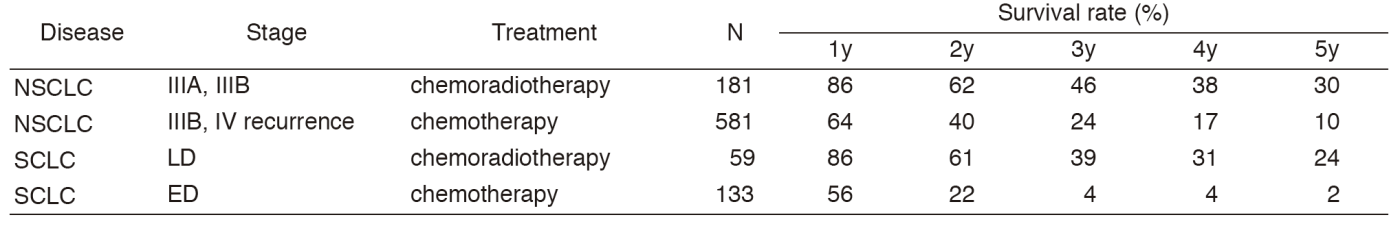
A total of 450 new patients were admitted in 2016, and the backgrounds and initial treatments of these patients are shown in Tables 1 and 2. The initial treatments were chemotherapy in 231, adjuvant chemotherapy after surgery in 58, chemoradiotherapy in 63, chemoradiotherapy followed by surgery in seven, chemotherapy followed by surgery in one, curative radiotherapy in seven, and supportive care including palliative radiotherapy in 36. Survival of lung cancer patients treated in 2007-2011 in our department is shown in Table 3.
Research activities
Research activities of the department can be classified into four categories: (1) multi-institutional phase III studies to establish new standard treatments for lung cancer; (2) phase I and phase II studies to evaluate new anticancer drugs, (3) pharmacokinetic and pharmacodynamic (PK/PD) studies to investigate interpatient variability, optimal administration schedules, and drug-drug interactions; and (4) translational research using clinical samples from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies.
Clinical trials
Our department is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments for lung cancer such as the Japan Clinical Oncology Group (JCOG) trials and global trials conducted by pharmaceutical companies. Three JCOG phase III studies, JCOG1201 for elderly ED-SCLC, JCOG1206 for high grade neuroendocrine carcinoma, and JCOG1210/WJOG7813L for elderly non-squamous NSCLC are ongoing. In addition to these studies, JCOG1404 (AGAIN), a phase III study for EGFR mutation positive NSCLC was started in December 2015. Our department is also participating in nationwide screening projects of lung cancer with rare driver mutation (LC-SCRUM) and phase II studies targeting rare driver mutation. Furthermore, our department carried out many clinical trials using 3rd generation EGFR-TKIs, anti-PD-1Ab, anti-PD-L1Ab.
Education
In 2016, three chief residents and 14 residents joined our department. A monthly research conference is held to discuss about clinical and translational research conducted by young doctors.
Future prospects
The recent progression of lung cancer treatment is very rapid. Driver gene alteration targeted therapy such as EGFR-TKIs and ALK inhibitors are already established as a standard treatment for lung cancer patients with EGFR mutation and ALK fusion gene. Other rare driver gene alterations such as ROS1 fusion, RET fusion, and BRAF mutation could be good targets for treatment of lung cancer. Immunotherapy using anti-PD-1Ab has been established as a standard 2nd or 3rd line treatment for NSCLC. Anti-PD-L1Ab will also be established as a standard treatment for lung cancer in the near future. These immunotherapies could provide durable response for some lung cancer patients. The establishment of good biomarkers to identify the patients who respond to immunotherapy is very important.
List of papers published in 2016
Journal
1.Nokihara H, Yamada Y, Fujiwara Y, Yamamoto N, Wakui H, Nakamichi S, Kitazono S, Inoue K, Harada A, Taube T, Takeuchi Y, Tamura T. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs, 34:66-74, 2016
2.Shimomura A, Fujiwara Y, Kondo S, Kodaira M, Iwasa S, Kitano S, Tanabe Y, Tamura K, Yamamoto N. Tremelimumab-associated tumor regression following after initial progression: two case reports. Immunotherapy, 8:9-15, 2016
3.Yamashita M, Kitano S, Aikawa H, Kuchiba A, Hayashi M, Yamamoto N, Tamura K, Hamada A. A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells. Sci Rep, 6:19772, 2016
4.Goto K, Endo M, Kusumoto M, Yamamoto N, Ohe Y, Shimizu A, Fukuoka M. Bevacizumab for non-small-cell lung cancer: A nested case control study of risk factors for hemoptysis. Cancer Sci, 107:1837-1842, 2016
5.Horinouchi H. The prospect of patritumab for treating non-small cell lung cancer. Expert Opin Biol Ther, 16:1549-1555, 2016
6.Horinouchi H. Role of multimodality therapy in cIIIA-N2 non-small cell lung cancer: perspective. Jpn J Clin Oncol, 46:1174-1178, 2016
7.Hida T, Nakagawa K, Seto T, Satouchi M, Nishio M, Hotta K, Takahashi T, Ohe Y, Takeda K, Tatsuno M, Asakawa T, Shimada T, Tanaka T, Tamura T. Pharmacologic study (JP28927) of alectinib in Japanese patients with ALK+ non-small-cell lung cancer with or without prior crizotinib therapy. Cancer Sci, 107:1642-1646, 2016
8.Noda S, Kanda S. Addressing epidermal growth factor receptor tyrosine kinase inhibitor resistance in non-small cell lung cancer. Expert Rev Respir Med, 10:547-556, 2016
9.Dittrich C, Kosty M, Jezdic S, Pyle D, Berardi R, Bergh J, El-Saghir N, Lotz J-P, Osterlund P, Pavlidis N, Purkalne G, Awada A, Banerjee S, Bhatia S, Bogaerts J, Buckner J, Cardoso F, Casali P, Chu E, Close JL, Coiffier B, Connolly R, Coupland S, De Petris L, De Santis M, de Vries EGE, Dizon DS, Duff J, Duska LR, Eniu A, Ernstoff M, Felip E, Fey MF, Gilbert J, Girard N, Glaudemans AWJM, Gopalan PK, Grothey A, Hahn SM, Hanna D, Herold C, Herrstedt J, Homicsko K, Jones DV, Jr., Jost L, Keilholz U, Khan S, Kiss A, Kohne C-H, Kunstfeld R, Lenz H-J, Lichtman S, Licitra L, Lion T, Litiere S, Liu L, Loehrer PJ, Markham MJ, Markman B, Mayerhoefer M, Meran JG, Michielin O, Moser EC, Mountzios G, Moynihan T, Nielsen T, Ohe Y, Oberg K, Palumbo A, Peccatori FA, Pfeilstocker M, Raut C, Remick SC, Robson M, Rutkowski P, Salgado R, Schapira L, Schernhammer E, Schlumberger M, Schmoll H-J, Schnipper L, Sessa C, Shapiro CL, Steele J, Sternberg CN, Stiefel F, Strasser F, Stupp R, Sullivan R, Tabernero J, Travado L, Verheij M, Voest E, Vokes E, Von Roenn J, Weber JS, Wildiers H, Yarden Y. ESMO / ASCO Recommendations for a Global Curriculum in Medical Oncology Edition 2016. ESMO Open, 1:e000097, 2016
10.Planchard D, Brown KH, Kim D-W, Kim S-W, Ohe Y, Felip E, Leese P, Cantarini M, Vishwanathan K, Janne PA, Ranson M, Dickinson PA. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother Pharmacol, 77:767-776, 2016
11.Horinouchi H. Anti-vascular endothelial growth factor therapies at the crossroads: linifanib for non-small cell lung cancer. Transl Lung Cancer Res, 5:78-81, 2016
12.Takeuchi K, Togashi Y, Kamihara Y, Fukuyama T, Yoshioka H, Inoue A, Katsuki H, Kiura K, Nakagawa K, Seto T, Maemondo M, Hida T, Harada M, Ohe Y, Nogami N, Yamamoto N, Nishio M, Tamura T. Prospective and clinical validation of ALK immunohistochemistry: results from the phase I/II study of alectinib for ALK-positive lung cancer (AF-001JP study). Ann Oncol, 27:185-192, 2016
13.Seki Y, Fujiwara Y, Kohno T, Takai E, Sunami K, Goto Y, Horinouchi H, Kanda S, Nokihara H, Watanabe S, Ichikawa H, Yamamoto N, Kuwano K, Ohe Y. Picoliter-Droplet Digital Polymerase Chain Reaction-Based Analysis of Cell-Free Plasma DNA to Assess EGFR Mutations in Lung Adenocarcinoma That Confer Resistance to Tyrosine-Kinase Inhibitors. Oncologist, 21:156-164, 2016
14.Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K, Tanaka H, Takeda K, Nishio M, Mori K, Satouchi M, Hida T, Yoshimura N, Kozuki T, Imamura F, Kiura K, Okamoto H, Sawa T, Tamura T. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol, 17:1147-1157, 2016
15.Shiraishi K, Okada Y, Takahashi A, Kamatani Y, Momozawa Y, Ashikawa K, Kunitoh H, Matsumoto S, Takano A, Shimizu K, Goto A, Tsuta K, Watanabe S, Ohe Y, Watanabe Y, Goto Y, Nokihara H, Furuta K, Yoshida A, Goto K, Hishida T, Tsuboi M, Tsuchihara K, Miyagi Y, Nakayama H, Yokose T, Tanaka K, Nagashima T, Ohtaki Y, Maeda D, Imai K, Minamiya Y, Sakamoto H, Saito A, Shimada Y, Sunami K, Saito M, Inazawa J, Nakamura Y, Yoshida T, Yokota J, Matsuda F, Matsuo K, Daigo Y, Kubo M, Kohno T. Association of variations in HLA class II and other loci with susceptibility to EGFR-mutated lung adenocarcinoma. Nat Commun, 7:12451, 2016
16.Horie M, Tamiya H, Goto Y, Suzuki M, Matsuzaki H, Hasegawa WT, Noguchi S, Kawakami M, Matsuta K, Nagase T, Sakamoto Y. Nonspecific elevation of serum Aspergillus galactomannan antigen levels in patients with rheumatoid arthritis. Respir Investig, 54:44-49, 2016
17.Ito T, Matsubara D, Tanaka I, Makiya K, Tanei Z, Kumagai Y, Shiu SJ, Nakaoka HJ, Ishikawa S, Isagawa T, Morikawa T, Shinozaki-Ushiku A, Goto Y, Nakano T, Tsuchiya T, Tsubochi H, Komura D, Aburatani H, Dobashi Y, Nakajima J, Endo S, Fukayama M, Sekido Y, Niki T, Murakami Y. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci, 107:1527-1538, 2016
18.Fujiwara Y, Hamada A, Mizugaki H, Aikawa H, Hata T, Horinouchi H, Kanda S, Goto Y, Itahashi K, Nokihara H, Yamamoto N, Ohe Y. Pharmacokinetic profiles of significant adverse events with crizotinib in Japanese patients with ABCB1 polymorphism. Cancer Sci, 107:1117-1123, 2016
19.Fujiwara Y, Tamura K, Kondo S, Tanabe Y, Iwasa S, Shimomura A, Kitano S, Ogasawara K, Turner PK, Mori J, Asou H, Chan EM, Yamamoto N. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol, 78:281-288, 2016
20.Funakoshi Y, Fujiwara Y, Kiyota N, Mukohara T, Shimada T, Toyoda M, Imamura Y, Chayahara N, Tomioka H, Umezu M, Otsuki N, Nibu K, Minami H. Validity of new methods to evaluate renal function in cancer patients treated with cisplatin. Cancer Chemother Pharmacol, 77:281-288, 2016
21.Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Sumi M, Tamura T, Ohe Y. Candidates for Intensive Local Treatment in cIIIA-N2 Non-Small Cell Lung Cancer: Deciphering the Heterogeneity. Int J Radiat Oncol Biol Phys, 94:155-162, 2016
22.Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Hozumi H, Tamura T. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol, 27:2242-2250, 2016
23.Kataoka T, Kiyota N, Shimada T, Funakoshi Y, Chayahara N, Toyoda M, Fujiwara Y, Nibu K, Komori T, Sasaki R, Mukohara T, Minami H. Randomized trial of standard pain control with or without gabapentin for pain related to radiation-induced mucositis in head and neck cancer. Auris Nasus Larynx, 43:677-684, 2016
24.Katsuya Y, Horinouchi H, Asao T, Kitahara S, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Watanabe S, Tsuta K, Ohe Y. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer, 99:4-10, 2016
25.Makino Y, Watanabe M, Makihara RA, Nokihara H, Yamamoto N, Ohe Y, Sugiyama E, Sato H, Hayashi Y. Simultaneous optimization of limited sampling points for pharmacokinetic analysis of amrubicin and amrubicinol in cancer patients. Asia Pac J Clin Oncol, 12:259-264, 2016
26.Minami H, Ando Y, Ma BBY, Hsiang Lee J, Momota H, Fujiwara Y, Li L, Fukino K, Ito K, Tajima T, Mori A, Lin C-C. Phase I, multicenter, open-label, dose-escalation study of sonidegib in Asian patients with advanced solid tumors. Cancer Sci, 107:1477-1483, 2016
27.Sunami K, Furuta K, Tsuta K, Sasada S, Izumo T, Nakaoku T, Shimada Y, Saito M, Nokihara H, Watanabe S, Ohe Y, Kohno T. Multiplex Diagnosis of Oncogenic Fusion and MET Exon Skipping by Molecular Counting Using Formalin-Fixed Paraffin Embedded Lung Adenocarcinoma Tissues. J Thorac Oncol, 11:203-212, 2016
28.Asao T, Fujiwara Y, Sunami K, Kitahara S, Goto Y, Kanda S, Horinouchi H, Nokihara H, Yamamoto N, Ichikawa H, Kohno T, Tsuta K, Watanabe S, Takahashi K, Ohe Y. Medical treatment involving investigational drugs and genetic profile of thymic carcinoma. Lung Cancer, 93:77-81, 2016
29.Mizugaki H, Yamamoto N, Murakami H, Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T, Takahashi T. Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumors. Invest New Drugs, 34:596-603, 2016
30.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, Abe T, Funakoshi T, Yamamoto N, Amagai M, Yamazaki N. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget, 7:77404-77415, 2016
31.Nokihara H, Yamamoto N, Ohe Y, Hiraoka M, Tamura T. Pharmacokinetics of Weekly Paclitaxel and Feasibility of Dexamethasone Taper in Japanese Patients with Advanced Non-small Cell Lung Cancer. Clin Ther, 38:338-347, 2016
32.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
33.Tamura Y, Nokihara H, Honda K, Tanabe Y, Asahina H, Yamada Y, Enatsu S, Kurek R, Yamamoto N, Tamura T. Phase I study of the second-generation, recombinant, human EGFR antibody necitumumab in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 78:995-1002, 2016
34.Tanabe Y, Ichikawa H, Kohno T, Yoshida H, Kubo T, Kato M, Iwasa S, Ochiai A, Yamamoto N, Fujiwara Y, Tamura K. Comprehensive screening of target molecules by next-generation sequencing in patients with malignant solid tumors: guiding entry into phase I clinical trials. Mol Cancer, 15:73, 2016
35.Yoh K, Hosomi Y, Kasahara K, Yamada K, Takahashi T, Yamamoto N, Nishio M, Ohe Y, Koue T, Nakamura T, Enatsu S, Lee P, Ferry D, Tamura T, Nakagawa K. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer, 99:186-193, 2016
36.Saruwatari K, Umemura S, Nomura S, Kirita K, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Ohe Y, Goto K. Prognostic Factor Analysis in Patients With Small-Cell Lung Cancer Treated With Third-Line Chemotherapy. Clin Lung Cancer, 17:581-587, 2016
37.Niho S, Nokihara H, Nihei K, Akimoto T, Sumi M, Ito Y, Yoh K, Goto K, Ohmatsu H, Horinouchi H, Yamamoto N, Sekine I, Kubota K, Ohe Y, Tamura T. Dose-Escalation Study of Thoracic Radiotherapy in Combination With Pemetrexed Plus Cisplatin in Japanese Patients With Locally Advanced Nonsquamous Non-Small Cell Lung Cancer: A Post Hoc Analysis of Survival and Recurrent Sites. Am J Clin Oncol, 39:132-135, 2016
38.Zenda S, Ota Y, Tachibana H, Ogawa H, Ishii S, Hashiguchi C, Akimoto T, Ohe Y, Uchitomi Y. A prospective picture collection study for a grading atlas of radiation dermatitis for clinical trials in head-and-neck cancer patients. J Radiat Res, 57:301-306, 2016
39.Tagami K, Kashiwase Y, Yokoyama A, Nishimura H, Miyano K, Suzuki M, Shiraishi S, Matoba M, Ohe Y, Uezono Y. The atypical antipsychotic, olanzapine, potentiates ghrelin-induced receptor signaling: An in vitro study with cells expressing cloned human growth hormone secretagogue receptor. Neuropeptides, 58:93-101, 2016
40.Miura N, Kamita M, Kakuya T, Fujiwara Y, Tsuta K, Shiraishi H, Takeshita F, Ochiya T, Shoji H, Huang W, Ohe Y, Yamada T, Honda K. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget, 7:33165-33178, 2016