HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Endoscopy, Gastrointestinal Endoscopy Division (the Endoscopy Center)
Yutaka Saito, Takahisa Matsuda, Ichiro Oda, Yasuo Kakugawa, Takeshi Nakajima, Shigetaka Yoshinaga, Haruhisa Suzuki, Satoru Nonaka, Taku Sakamoto, Seiichiro Abe, Masayoshi Yamada,
Masau Sekiguchi and Hiroyuki Takamaru (Gastrointestinal Endoscopy, National Cancer Center Hospital)
Yuji Matsumoto and Takaaki Tsuchida (Respiratory Endoscopy)
Introduction
The Department of Endoscopy moved to the New Endoscopy Center on 20th January, 2014, and we believe this is currently the biggest Endoscopy Center in Japan (15 Endoscopy Rooms (251.112m2) and 136.788m2 Recovery Rooms on two floors of 1,949.554m2).
The total number of nursing staff increased to 15 and three endoscopy engineers are working with us.
The Gastrointestinal Endoscopy Division has 13 staff physicians in the National Cancer Center Hospital (NCCH) and in the Division of Screening Technology of the Center for Public Health Sciences, four chief residents, nine residents, one trainee, and several rotating residents.
The Respiratory Endoscopy Division has three staff members, and the total number of bronchoscopies and therapeutic procedures has been dramatically increased.
Dramatic developments have recently changed the operational mechanism and design of endoscopes along with a variety of accessory devices and instruments, so clinical applications using the latest equipment are evolving on a continuous basis. In the Gastrointestinal Endoscopy Division, more advanced and technically difficult endoscopic treatments such as endoscopic submucosal dissection (ESD) are being used in place of conventional endoscopic mucosal resection (EMR) not only for early gastric cancer, but also for superficial esophageal and colorectal neoplasms. In addition, educational activities are an important part of our division's activities with many Japanese medical students, residents, and staff physicians as well as approximately 100 overseas post-graduate physicians attending our training courses annually.
Routine activities in Gastrointestinal (GI) Endoscopy
Various diagnostic techniques including chromoendoscopy, magnifying endoscopy, and endoscopic ultrasonography (EUS) are used to detect and evaluate early malignant lesions. Capsule endoscopy has also been accepted as being far less invasive. In our facility, small intestine capsule endoscopy has been performed since 2005. In order to obtain a more accurate endoscopic diagnosis of gastrointestinal disease, we routinely use the recently developed narrow-band imaging (NBI) system. A total of 12,597, 4,391, 482, 78, 224, 82, and 130 screening and/or diagnostic procedures by gastroscopy, colonoscopy, EUS, EUS-fine needle aspiration (EUS-FNA), endoscopic retrograde cholangiopancreatography (ERCP), capsule endoscopy, and double balloon endoscopies, respectively, was performed in 2016 (Table 1).
Due to the increasing number of patients with superficial gastrointestinal neoplasms, the number of therapeutic endoscopy procedures is also increasing in this field. In 2016, 2,958 endoscopic resections were carried out (pharynx 3, esophagus 211, stomach 388, duodenum 36, and colon 2,320). Among these, ESD, which was developed for large en-bloc resections with a low-risk of local recurrence, was performed for 125 superficial esophageal cancers, 388 early gastric cancers and 214 superficial colorectal neoplasms. For colorectal ESDs and some esophageal ESDs, the newly developed ball-tip bipolar needle knife (Jet B-knife) and IT-knife nano were used together with CO2 insufflation. Our colleagues originally developed these procedures and devices.
ESD achieves a higher en-bloc resection rate compared to the standard EMR technique and is less invasive than a surgical operation while EUS-FNA provides a less invasive procedure to improve diagnosis for patients with pancreatic tumors, lymph-node swelling, submucosal tumors of the GI tract, etc.
Image-reading conferences are held regularly and we attend all clinical conferences in the Surgery, Oncology, Radiology, and Pathology Divisions to discuss and decide on treatment strategies.
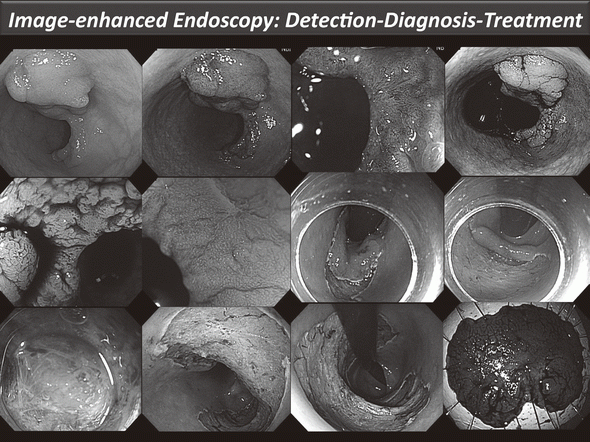
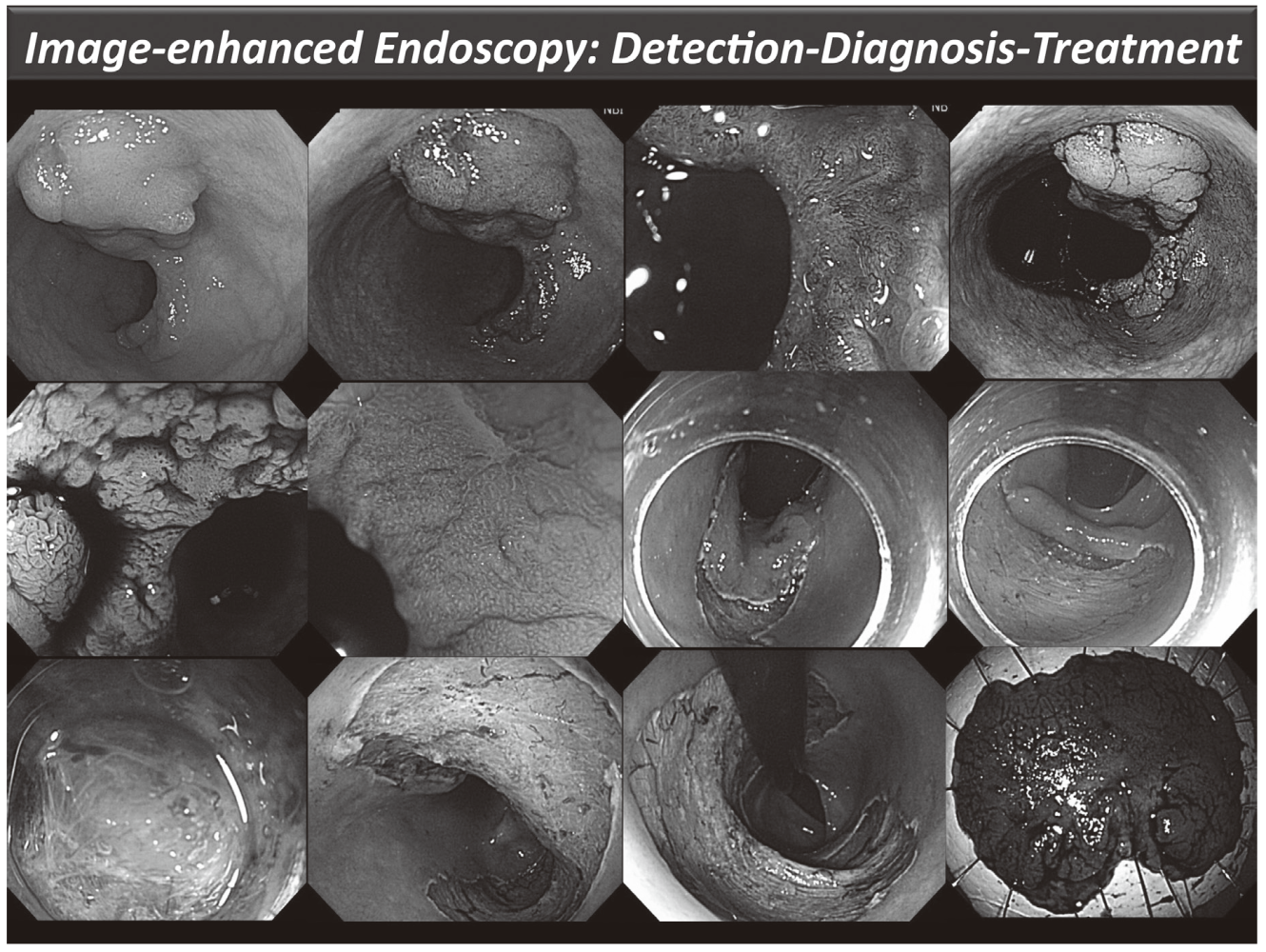
Clinical activities in GI Endoscopy (Figure 1)
Our efforts have been focused on new diagnostic and therapeutic strategies. For more accurate endoscopic diagnosis of gastrointestinal disease, we are utilizing the NBI system that enables us to narrow the spectral transmittance bandwidth of the optical filters used in the light source of electronic endoscope systems. In addition, we have conducted a trial study on an autofluorescence imaging (AFI) system. This system can identify lesions based on differences in tissue fluorescence properties and reveal gastrointestinal neoplasms that are not detectable with conventional endoscopy.
Figure 1. Endoscopic diagnosis using image-enhanced endoscopy (high-resolution endoscopy, narrow-band imaging and chromoscopy) and endoscopic submucosal dissection (ESD) procedure for treating early colon cancer
Clinical trials in GI Endoscopy
We have organized several multicenter study groups in order to evaluate the efficacy and clinical impact of newly developed endoscopies and medical devices prospectively.
Esophagus
We are currently enrolling our patients in two multicenter randomized controlled trials (RCTs). First, a phase II/III study has been introduced to compare endoscopic balloon dilatation combined with steroids to radial incision and cutting combined with steroids for refractory anastomotic stricture after esophagectomy (JCOG 1207: RICS study). Second, a phase III study is ongoing to compare oral steroid administration to local steroid injection therapy for the prevention of esophageal stricture after endoscopic submucosal dissection (JCOG1217: Steroid EESD P3).
In collaboration with TWIns (Tokyo Women's Medical University - Waseda University Joint Institution for Advanced Biomedical Sciences), we are conducting a clinical trial of cell sheet-based regenerative medicine, which could reduce complications such as severe stenosis and perforation related to intensive balloon dilations. This cell sheet-based regenerative medicine is one of the innovations in the gastrointestinal field and we believe that the cell-based regenerative medicine would be useful to improve the quality of life for patients after esophageal ESD.
Stomach
A nationwide cancer registry system has been developed for early gastric cancer treated with EMR/ESD. A five-year multicenter prospective cohort study has been ongoing using this cancer registry system since 2010 (J-WEB/EGC).
In a recent translational study, it was shown that Helicobacter pylori (H. pylori) infection induces methylation of CpG islands in non-cancerous mucosae and the methylation level in H. pylori-negative patients is closely associated with the risk of gastric cancer. A multicenter prospective observational study has confirmed the usefulness of the methylation level as a risk marker for metachronous gastric cancer after EMR/ESD followed by H. pylori eradication. Since 2015, a multicenter prospective observational study has been started to demonstrate the usefulness of the methylation level as a risk marker for gastric cancer developing after H. pylori eradication in healthy people. In addition, we have completed two multicenter RCTs. First one is a CONNECT-G trial to investigate the usefulness of endo-clip connecting dental floss (DFC) during gastric ESD that have a potential efficacy making a better view by traction with DFC. Second one is an RCT to compare the second generation NBI with white-light imaging (WLI) for detection of early gastric cancer (EGC Detection Trial).
Pancreas
We prospectively evaluated the efficacy and safety of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for pancreatic solid lesions in multicenters in Japan. This study was designed as a prospective cohort study conducted at the following five hospitals in Japan: the NCCH, Tokyo Medical University, Aichi Cancer Center Hospital, Gifu University Hospital, and Fukushima Medical University Aizu Medical Center. Two hundred and forty nine patients were enrolled from November 2011 to June 2013. Diagnostic sensitivity of EUS-FNA in this study was 97.2%. Diagnostic specificity, accuracy, positive predictive value, and negative predictive value were 88.0%, 96.2%, 100%, 81.4%, respectively. Complication after seven days was 1.6%. We could confirm that the efficacy and safety of EUS-FNA for pancreatic solid lesions is quite satisfied.
Colorectum
RCTs concerning colorectal neoplasms are ongoing as well.
The Japan Polyp Study (JPS), a multicenter randomized controlled trial conducted at 11 participating centers was initiated in 2003. Patients were eligible if they had two complete colonoscopies (1st-CS and 2nd-CS: interval; one year) with the removal of all neoplastic lesions. The aim of this study was to assess whether follow-up colonoscopy using high-definition colonoscope at three years as well as at both one and three years would detect important lesions including non-polypoid colorectal neoplasia. Moreover, we hypothesized that two complete colonoscopies may lengthen the surveillance interval more than 3 years after polypectomy in some selected patients. The JPS will provide preexisting comorbidity data, including the prevalence of both flat and depressed colorectal lesions, the quality of colonoscopy, and the risk of colorectal cancer (CRC). Furthermore, the study will clarify the long-term impact of colonoscopic removal on mortality due to CRC. The evidence will enable to elaborate the fundamental basis for the updated Japanese surveillance guidelines (Participants of JPS: 3,926, JPS Cohort: 1,288).
Little is known about the long-term outcomes of patients with submucosal invasive CRC who undergo endoscopic or surgical resection. We performed a retrospective analysis of long-term outcomes of patients treated for submucosal colon and rectal cancer. We collected data from 549 patients with submucosal colon cancer and 209 with submucosal rectal cancer who underwent endoscopic or surgical resection at six institutions, over a median follow-up period of 60.5 months. We assessed recurrence rates, five-year disease-free survival, and five-year overall survival. As a result of patients treated with only endoscopic resection, the risk for local recurrence was significantly higher in high-risk patients with submucosal rectal cancer than patients with submucosal colon cancer. The addition of surgery is therefore recommended for patients with submucosal rectal cancer with pathology features indicating a high risk of tumor progression (Gastroenterology 2012). Considering this study result, we have just started a prospective cohort study for the possibility of chemo-radiotherapy for high-risk rectal submucosal cancer after endoscopic resections.
A nationwide cancer registry system has also been developed for early CRC treated with ESD. A five-year multicenter prospective cohort study has been ongoing using this cancer registry system since 2013. A total of 2,066 patients was enrolled to this multicenter cohort study and this should be the largest cohort study in colorectal ESD in the world.
A multicenter RCT to compare the detectability of colorectal neoplastic lesions using a novel endoscopic system with blue-laser imaging (BLI). A newly developed system that uses BLI improves the detection of adenomatous lesions compared with WLI (Clinical trial registration number: UMIN 000014555.) and will be published in Gastrointest Endosc. 2017 Jan 29.
The other multicenter RCT to compare the adenoma miss rate between new full-spectrum endoscopy (Fuse) and conventional colonoscopy was just completed and data analysis is ongoing.
A multicenter RCT to clarify the efficacy of autofluorescence imaging (AFI) on flat adenoma detection is also ongoing (A-FLAT trial).
Molecular and fluorescence imaging and Database study
Molecular imaging endoscopy is one of a new era for very early cancer diagnosis and detection of metastasis. We have just started a collaborative study with the Departments of Endoscopy, Colorectal Surgery, Gastric Surgery, Pathology and Clinical Laboratories, the NCC-Research Institute, the University of Tokyo, and the Jikei University School of Medicine.
Probe-based confocal laser endomicroscopy (pCLE) allows real-time, in vivo high resolution imaging of the gastrointestinal epithelium at a cellular level. We are going to conduct a multicenter prospective study supported by the Japan Gastroenterological Endoscopy Society (JGES) to evaluate diagnostic yield of pCLE for gastric neoplasms.
We have been collaborating with the Japan Gastroenterological Endoscopy Society (JGES) in order to build a Japan endoscopy database (JED) of gastrointestinal endoscopies including not only therapeutic but also diagnostic procedures. This all Japan project is named JED and has the potential to construct the largest and most precise database of all endoscopic procedures. Japanese endoscopists are well known as most excellent endoscopists, therefore, we can create lots of evidences using this huge endoscopy database from now.
Research and development of new endoscopy using artificial intelligence
The recent development of artificial intelligence (AI) using deep learning is expected to apply in precision medicine. We have been researching and developing new endoscopic systems using AI. First, in order to support endoscopist's detection of CRC and precancerous lesion during colonoscopy by AI, we have been researching and developing a software program using convolutional neural networks based on mathematical morphology and hardware, which works in real-time. We will extend this system to genomic data and other gastrointestinal organs, such as the stomach and esophagus, in the future.
Second, we have started a multicenter image analyzing study to compare a new automatic diagnosis system using AI with endoscopist diagnosis of colorectal lesion using endocytoscopy.
List of papers published in 2016
Journal
1.Sekiguchi M, Igarashi A, Matsuda T, Matsumoto M, Sakamoto T, Nakajima T, Kakugawa Y, Yamamoto S, Saito H, Saito Y. Optimal use of colonoscopy and fecal immunochemical test for population-based colorectal cancer screening: a cost-effectiveness analysis using Japanese data. Jpn J Clin Oncol, 46:116-125, 2016
2.Chiu H-M, Ching JYL, Wu KC, Rerknimitr R, Li J, Wu D-C, Goh KL, Matsuda T, Kim H-S, Leong R, Yeoh KG, Chong VH, Sollano JD, Ahmed F, Menon J, Sung JJY. A Risk-Scoring System Combined With a Fecal Immunochemical Test Is Effective in Screening High-Risk Subjects for Early Colonoscopy to Detect Advanced Colorectal Neoplasms. Gastroenterology, 150:617-625 e613, 2016
3.Matsumoto M, Nakajima T, Kakugawa Y, Sakamoto T, Kuribayashi S, Otake Y, Matsuda T, Kanemitsu Y, Taniguchi H, Saito Y. Surveillance using capsule endoscopy is safe in post-colectomy patients with familial adenomatous polyposis: a prospective Japanese study. Fam Cancer, 15:75-83, 2016
4.Pioche M, Matsumoto M, Takamaru H, Sakamoto T, Nakajima T, Matsuda T, Abe S, Kakugawa Y, Otake Y, Saito Y. Endocuff-assisted colonoscopy increases polyp detection rate: a simulated randomized study involving an anatomic colorectal model and 32 international endoscopists. Surg Endosc, 30:288-295, 2016
5.Watabe H, Nakamura T, Yamada A, Kakugawa Y, Nouda S, Terano A. Assessment of an electronic learning system for colon capsule endoscopy: a pilot study. J Gastroenterol, 51:579-585, 2016
6.Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, Shimizu Y, Doyama H, Koike T, Takizawa K, Hirao M, Okada H, Yoshii T, Konishi K, Yamanouchi T, Tsuda T, Omori T, Kobayashi N, Shimoda T, Ochiai A, Amanuma Y, Ohashi S, Matsuda T, Ishikawa H, Yokoyama A, Muto M. Alcohol Consumption and Multiple Dysplastic Lesions Increase Risk of Squamous Cell Carcinoma in the Esophagus, Head, and Neck. Gastroenterology, 151:860-869 e867, 2016
7.Saito Y. La resezione en bloc delle neoplasia colorettali superficiali. Giornale italiano di endoscopia digestiva, 14-18, 2016
8.Sano Y, Tanaka S, Kudo S-E, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu K-I, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano H-O, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc, 28:526-533, 2016
9.Matsuda T, Oka S, Ikematsu H, Matsushita H-o, Mori Y, Takeuchi Y, Tamai N, Kawamura T, Chino A, Keum B, Khomvilai S, Uraoka T. Endoscopic diagnosis of colorectal serrated lesions: Current status and future perspectives based on the results of a questionnaire survey. Dig Endosc, 28 Suppl 1:35-42, 2016
10.Kakushima N, Hori K, Ono H, Horimatsu T, Uedo N, Ohata K, Doyama H, Kaneko K, Oda I, Hikichi T, Kawahara Y, Niimi K, Takaki Y, Mizuno M, Yazumi S, Hosokawa A, Imagawa A, Niimi M, Yoshimura K, Muto M. Proton pump inhibitor after endoscopic resection for esophageal squamous cell cancer: multicenter prospective randomized controlled trial. J Gastroenterol, 51:104-111, 2016
11.Inoki K, Nakajima T, Sekine S, Sugano K, Tsukamoto S, Yamada M, Mutoh M, Sakamoto T, Matsuda T, Sekiguchi M, Ushiama M, Yoshida T, Sakamoto H, Kanemitsu Y, Saito Y. Depressed-type submucosal invasive colorectal cancer in a patient with Lynch syndrome diagnosed using short-interval colonoscopy. Dig Endosc, 28:749-754, 2016
12.Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M, Ushijima T, Oda I. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer, 19:911-918, 2016
13.Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer, 19:198-205, 2016
14.Tsuruki ES, Saito Y, Abe S, Takamaru H, Yamada M, Sakamoto T, Nakajima T, Matsuda T, Sekine S, Taniguchi H. Evaluating the efficacy and safety of a novel endoscopic fluorescence imaging modality using oral 5-aminolevulinic acid for colorectal tumors. Endosc Int Open, 4:E30-35, 2016
15.Iacopini F, Saito Y, Gotoda T, Grossi C, Costamagna G. Endoscopic submucosal dissection of a nonpolypoid superficial neoplasm of the terminal ileum. Endoscopy, 48 Suppl 1:E57-58, 2016
16.Yamada M, Saito Y, Sakamoto T, Nakajima T, Kushima R, Parra-Blanco A, Matsuda T. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy, 48:456-464, 2016
17.Perez-Cuadrado Robles E, Yamada M, Saito Y. Successful balloon overtube-guided colorectal endoscopic submucosal dissection by a gastroscope. Rev Esp Enferm Dig, 108:280-281, 2016
18.Mori Y, Kudo S-e, Ogawa Y, Wakamura K, Kudo T, Misawa M, Hayashi T, Katagiri A, Miyachi H, Inoue H, Oka S, Matsuda T. Diagnosis of sessile serrated adenomas/polyps using endocytoscopy (with videos). Dig Endosc, 28 Suppl 1:43-48, 2016
19.Matsuda T, Chiu H-M, Sano Y, Fujii T, Ono A, Saito Y. Surveillance colonoscopy after endoscopic treatment for colorectal neoplasia: From the standpoint of the Asia-Pacific region. Dig Endosc, 28:342-347, 2016
20.De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol, 104:138-155, 2016
21.Bhatt A, Abe S, Kumaravel A, Parsi MA, Stevens T, Jang S, Lopez R, Oda I, Vargo JJ, Saito Y. Video-based supervision for training of endoscopic submucosal dissection. Endoscopy, 48:711-716, 2016
22.Iacopini F, Gotoda T, Elisei W, Rigato P, Montagnese F, Saito Y, Costamagna G, Iacopini G. Heterotopic gastric mucosa in the anus and rectum: first case report of endoscopic submucosal dissection and systematic review. Gastroenterol Rep (Oxf), 4:196-205, 2016
23.Takamaru H, Saito Y, Yamada M, Tsuruki ES, Kinjo Y, Otake Y, Sakamoto T, Nakajima T, Matsuda T. Clinical impact of endoscopic clip closure of perforations during endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc, 84:494-502 e491, 2016
24.Sasaki A, Nakajima T, Egashira H, Takeda K, Tokoro S, Ichita C, Masuda S, Uojima H, Koizumi K, Kinbara T, Sakamoto T, Saito Y, Kako M. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol, 22:2636-2641, 2016
25.Tokuda T, Arai Y, Sone M, Sugawara S, Morita S, Saito Y. Coil Embolization for the Treatment of Esophageal Perforation after Endoscopic Submucosal Dissection. J Vasc Interv Radiol, 27:1461-1463, 2016
26.Inoki K, Sakamoto T, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Saito Y. Successful endoscopic closure of a colonic perforation one day after endoscopic mucosal resection of a lesion in the transverse colon. World J Clin Cases, 4:238-242, 2016
27.Wong MCS, Ching JYL, Chiu H-M, Wu KC, Rerknimitr R, Li J, Wu D-C, Goh KL, Matsuda T, Kim H-S, Leong R, Yeoh KG, Chong VH, Sollano JD, Ahmed F, Menon J, Ng SC, Wu JCY, Chan FKL, Sung JJY. Risk of Colorectal Neoplasia in Individuals With Self-Reported Family History: A Prospective Colonoscopy Study from 16 Asia-Pacific Regions. Am J Gastroenterol, 111:1621-1629, 2016
28.Abe S, Sakamoto T, Takamaru H, Yamada M, Nakajima T, Matsuda T, Saito Y. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc, 30:5459-5464, 2016
29.Sekiguchi M, Kakugawa Y, Terauchi T, Matsumoto M, Saito H, Muramatsu Y, Saito Y, Matsuda T. Sensitivity of 2-[18F]fluoro-2-deoxyglucose positron emission tomography for advanced colorectal neoplasms: a large-scale analysis of 7505 asymptomatic screening individuals. J Gastroenterol, 51:1122-1132, 2016
30.Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, Sekine S, Kushima R, Katai H. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol, 51:961-970, 2016
31.Suzuki H, Oda I, Sekiguchi M, Abe S, Nonaka S, Yoshinaga S, Saito Y. Factors associated with incomplete gastric endoscopic submucosal dissection due to misdiagnosis. Endosc Int Open, 4:E788-793, 2016
32.Oda I, Suzuki H, Yoshinaga S. Endoscopic Submucosal Dissection for Early Gastric Cancer: Getting It Right!. Adv Exp Med Biol, 908:317-330, 2016
33.Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc, 28:3-15, 2016
34.Takamaru H, Yamada M, Sakamoto T, Nakajima T, Saito Y, Kakugawa Y, Matsumoto M, Matsuda T, Ide D, Saito S, Gulati S, Tajiri H. Dual camera colon capsule endoscopy increases detection of colorectal lesions. Scand J Gastroenterol, 51:1532-1533, 2016