HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Hepatobiliary and Pancreatic Oncology
Takuji Okusaka, Hideki Ueno, Chigusa Morizane, Shunsuke Kondo, Yasunari Sakamoto
Introduction
The Department of Hepatobiliary and Pancreatic Oncology treats tumors originating from the liver, biliary system, or pancreas, which include hepatocellular carcinoma (HCC), biliary tract cancer and pancreatic cancer. As part of the multi-disciplinary care given at the National Cancer Center Hospital (NCCH), we work closely with surgeons and radiologists who have special expertise in these areas. We also conduct research on the pathophysiology of hepatobiliary and pancreatic tumors, and seek to develop new and more effective diagnostic methods and treatments.
Our team and what we do
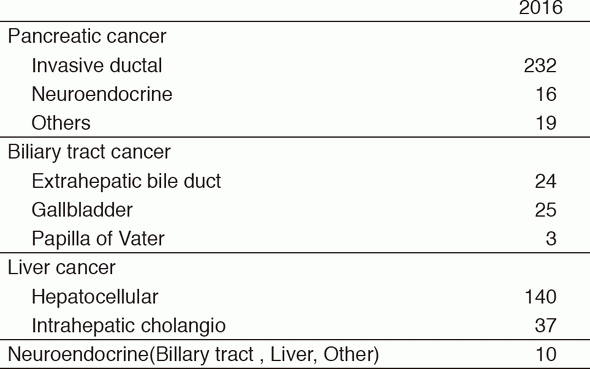
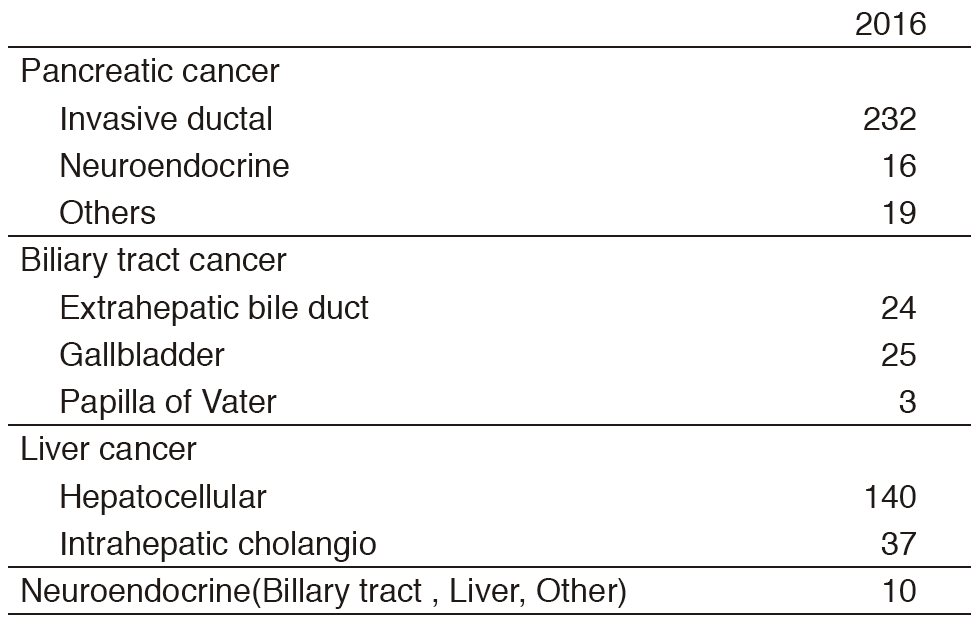
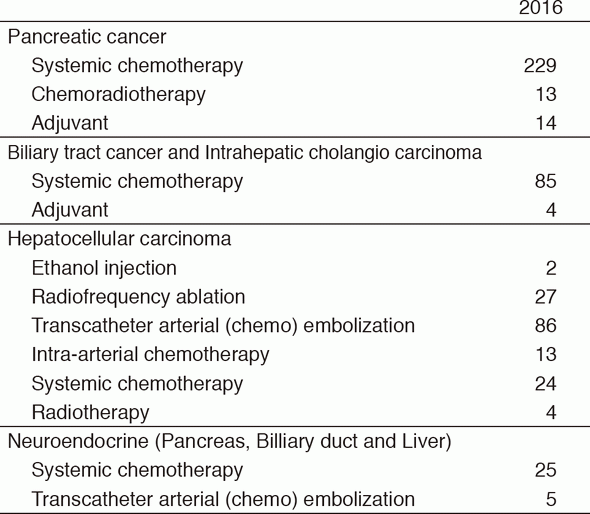
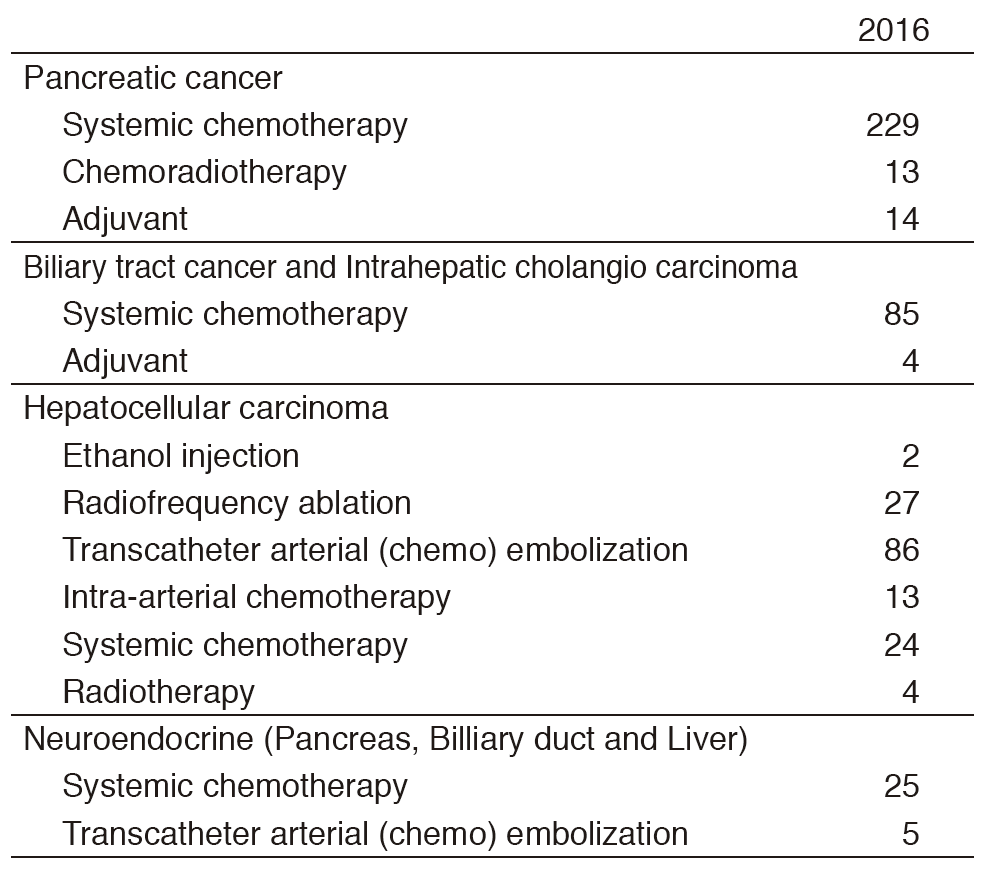
Our department consists of five staff oncologists and three to four residents. We have used percutaneous ablation therapy as a valuable alternative to surgery for most patients with three or fewer HCC nodules, all of which are smaller than 3 cm in diameter. We also perform transcatheter arterial chemoembolization (TACE), mainly in patients with multiple HCC nodules. Systemic or intra-arterial chemotherapeutic regimens are indicated in advanced HCC patients for whom locoregional intervention and surgery are unsuitable or unsuccessful. In patients with unresectable pancreatic cancer or biliary tract cancer, chemotherapy is performed in clinical practice or as a clinical trial to develop active treatment (Tables 1 & 2).
Research activities
We conducted a phase I, dose-escalation study evaluating the safety, preliminary efficacy, and pharmacokinetics of nintedanib, a triple angiokinase inhibitor, in Japanese patients with advanced hepatocellular carcinoma and mild/moderate liver impairment (Okusaka, Cancer Sci; 107:1791-1799). No dose-limiting toxicities were reported and the maximum tolerated dose for both groups was 200 mg twice daily. Nintedanib showed a manageable safety profile and efficacy signals, including in patients previously treated with sorafenib.
Another phase I study was conducted to determine the recommended dose of combining S-1 with gemcitabine and cisplatin for advanced biliary tract adenocarcinoma first-line therapy (Shoji, Jpn J Clin Oncol;46:132-7). This triweekly triplet regimen was well tolerated and showed promising antitumor activity. We recommend level 0, gemcitabine at 800 mg/m(2)/week, cisplatin at 25 mg/m(2)/week, and S-1 at 40 mg/m(2)/day during a 21-day cycle, in further studies under this schedule.
We investigated clinicopathologic features and the genetic background of pancreatic ductal adenocarcinoma occurring in young patients (age ≤ 40 years) to determine whether any difference exists in comparison with those of older patients (>40 years). Among 908 patients, a total of 17 were diagnosed at ages younger than 40. There were no other significant differences between young and old groups in terms of sex, smoking history, tumor location, Union for International Cancer Control stage, family histories of any cancer and PDAC in first-degree relatives, and medical history of other cancer. Targeted sequencing analysis demonstrated no definite "pathogenic" variants.
Clinical trials
Thirty-one clinical trials are ongoing, including eighteen phase I or I/II trials, four phase II or II/III trials, and four phase III trials such as adjuvant chemotherapy after resection versus resection alone for patients with resectable tumor, and chemotherapy with a new regimen versus standard therapy for patients with advanced tumor. Our studies are supported by the National Cancer Center (NCC) Research and Development Fund (Grant No. 26-A-4), Health and Labour Sciences Research Grants (Project for Development of Innovative Research on Cancer Therapeutics -075, -080, -141) from the Japan Agency for Medical Research and Development.
Education
Our staff members are working closely with residents to support their skill development and knowledge expansion in both clinical and research fields. We are conducting conferences daily for clinical practice and weekly for research development. The residents in our department have published three papers as first authors in peer-reviewed journals in 2016, and are performing twelve ongoing studies as leading researchers, with assistance from staff members.
Future prospects
Our department keeps providing the best and latest diagnosis, treatment and supportive care, and developing more effective methods and techniques for all patients with hepatobiliary and pancreatic cancers in this country and all over the world. Among them, conducting clinical trials with novel promising agents for these diseases is considered one of the most important tasks, and the establishment of cutting-edge medical treatments in this field is the most significant mission for us. To achieve our aim, we are ongoing screening for patients with gene-mutations, and are planning to conduct clinical trials for new molecular agents targeting these mutations.
List of papers published in 2016
Journal
1.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Okuyama H, Ueno H, Morizane C, Kondo S, Sakamoto Y, Okusaka T, Ochiai A. C-Reactive Protein Level Is an Indicator of the Aggressiveness of Advanced Pancreatic Cancer. Pancreas, 45:110-116, 2016
2.Shoji H, Morizane C, Sakamoto Y, Kondo S, Ueno H, Takahashi H, Ohno I, Shimizu S, Mitsunaga S, Ikeda M, Okusaka T. Phase I clinical trial of oral administration of S-1 in combination with intravenous gemcitabine and cisplatin in patients with advanced biliary tract cancer. Jpn J Clin Oncol, 46:132-137, 2016
3.Akizuki N, Shimizu K, Asai M, Nakano T, Okusaka T, Shimada K, Inoguchi H, Inagaki M, Fujimori M, Akechi T, Uchitomi Y. Prevalence and predictive factors of depression and anxiety in patients with pancreatic cancer: a longitudinal study. Jpn J Clin Oncol, 46:71-77, 2016
4.Ueda R, Narumi K, Hashimoto H, Miyakawa R, Okusaka T, Aoki K. Interaction of natural killer cells with neutrophils exerts a significant antitumor immunity in hematopoietic stem cell transplantation recipients. Cancer Med, 5:49-60, 2016
5.Fujimoto A, Furuta M, Totoki Y, Tsunoda T, Kato M, Shiraishi Y, Tanaka H, Taniguchi H, Kawakami Y, Ueno M, Gotoh K, Ariizumi S, Wardell CP, Hayami S, Nakamura T, Aikata H, Arihiro K, Boroevich KA, Abe T, Nakano K, Maejima K, Sasaki-Oku A, Ohsawa A, Shibuya T, Nakamura H, Hama N, Hosoda F, Arai Y, Ohashi S, Urushidate T, Nagae G, Yamamoto S, Ueda H, Tatsuno K, Ojima H, Hiraoka N, Okusaka T, Kubo M, Marubashi S, Yamada T, Hirano S, Yamamoto M, Ohdan H, Shimada K, Ishikawa O, Yamaue H, Chayama K, Miyano S, Aburatani H, Shibata T, Nakagawa H. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet, 48:500-509, 2016
6.Yachida S, Wood LD, Suzuki M, Takai E, Totoki Y, Kato M, Luchini C, Arai Y, Nakamura H, Hama N, Elzawahry A, Hosoda F, Shirota T, Morimoto N, Hori K, Funazaki J, Tanaka H, Morizane C, Okusaka T, Nara S, Shimada K, Hiraoka N, Taniguchi H, Higuchi R, Oshima M, Okano K, Hirono S, Mizuma M, Arihiro K, Yamamoto M, Unno M, Yamaue H, Weiss MJ, Wolfgang CL, Furukawa T, Nakagama H, Vogelstein B, Kiyono T, Hruban RH, Shibata T. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell, 29:229-240, 2016
7.Takai E, Yachida S, Shimizu K, Furuse J, Kubo E, Ohmoto A, Suzuki M, Hruban RH, Okusaka T, Morizane C, Furukawa T. Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget, 7:74227-74235, 2016
8.Ohmoto A, Yachida S, Kubo E, Takai E, Suzuki M, Shimada K, Okusaka T, Morizane C. Clinicopathologic Features and Germline Sequence Variants in Young Patients (≤40 Years Old) With Pancreatic Ductal Adenocarcinoma. Pancreas, 45:1056-1061, 2016
9.Mizusawa J, Morizane C, Okusaka T, Katayama H, Ishii H, Fukuda H, Furuse J. Randomized Phase III study of gemcitabine plus S-1 versus gemcitabine plus cisplatin in advanced biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1113, FUGA-BT). Jpn J Clin Oncol, 46:385-388, 2016
10.Yoneyama T, Ohtsuki S, Honda K, Kobayashi M, Iwasaki M, Uchida Y, Okusaka T, Nakamori S, Shimahara M, Ueno T, Tsuchida A, Sata N, Ioka T, Yasunami Y, Kosuge T, Kaneda T, Kato T, Yagihara K, Fujita S, Huang W, Yamada T, Tachikawa M, Terasaki T. Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics. PLoS One, 11:e0161009, 2016
11.Okusaka T, Otsuka T, Ueno H, Mitsunaga S, Sugimoto R, Muro K, Saito I, Tadayasu Y, Inoue K, Loembe A-B, Ikeda M. Phase I study of nintedanib in Japanese patients with advanced hepatocellular carcinoma and liver impairment. Cancer Sci, 107:1791-1799, 2016
12.Ikeda M, Yamamoto H, Kaneko M, Oshima H, Takahashi H, Umemoto K, Watanabe K, Hashimoto Y, Ohno I, Mitsunaga S, Okusaka T. Screening rate for hepatitis B virus infection in patients undergoing chemotherapy in Japan. Int J Clin Oncol, 21:1162-1166, 2016
13.Ueno M, Okusaka T, Omuro Y, Isayama H, Fukutomi A, Ikeda M, Mizuno N, Fukuzawa K, Furukawa M, Iguchi H, Sugimori K, Furuse J, Shimada K, Ioka T, Nakamori S, Baba H, Komatsu Y, Takeuchi M, Hyodo I, Boku N. A randomized phase II study of S-1 plus oral leucovorin versus S-1 monotherapy in patients with gemcitabine-refractory advanced pancreatic cancer. Ann Oncol, 27:502-508, 2016
14.Morofuji N, Ojima H, Hiraoka N, Okusaka T, Esaki M, Nara S, Shimada K, Kishi Y, Kondo T. Antibody-based proteomics to identify an apoptosis signature for early recurrence of hepatocellular carcinoma. Clin Proteomics, 13:28, 2016
15.Kudo M, Ikeda M, Takayama T, Numata K, Izumi N, Furuse J, Okusaka T, Kadoya M, Yamashita S, Ito Y, Kokudo N. Safety and efficacy of sorafenib in Japanese patients with hepatocellular carcinoma in clinical practice: a subgroup analysis of GIDEON. J Gastroenterol, 51:1150-1160, 2016
16.Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, Sugimoto R, Tahara T, Ohmura T, Yasui K, Sato K, Ishii H, Furuse J, Okusaka T. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: randomized phase II trial. Ann Oncol, 27:2090-2096, 2016
17.Kimbara S, Kondo S. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J Gastroenterol, 22:7440-7452, 2016
18.Bridgewater J, Lopes A, Wasan H, Malka D, Jensen L, Okusaka T, Knox J, Wagner D, Cunningham D, Shannon J, Goldstein D, Moehler M, Bekaii-Saab T, McNamara MG, Valle JW. Prognostic factors for progression-free and overall survival in advanced biliary tract cancer. Ann Oncol, 27:134-140, 2016
19.Abou-Alfa GK, Puig O, Daniele B, Kudo M, Merle P, Park J-W, Ross P, Peron J-M, Ebert O, Chan S, Poon TP, Colombo M, Okusaka T, Ryoo B-Y, Minguez B, Tanaka T, Ohtomo T, Ukrainskyj S, Boisserie F, Rutman O, Chen Y-C, Xu C, Shochat E, Jukofsky L, Reis B, Chen G, Di Laurenzio L, Lee R, Yen C-J. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J Hepatol, 65:289-295, 2016
20.Fujimoto A, Furuta M, Totoki Y, Tsunoda T, Kato M, Shiraishi Y, Tanaka H, Taniguchi H, Kawakami Y, Ueno M, Gotoh K, Ariizumi S-I, Wardell CP, Hayami S, Nakamura T, Aikata H, Arihiro K, Boroevich KA, Abe T, Nakano K, Maejima K, Sasaki-Oku A, Ohsawa A, Shibuya T, Nakamura H, Hama N, Hosoda F, Arai Y, Ohashi S, Urushidate T, Nagae G, Yamamoto S, Ueda H, Tatsuno K, Ojima H, Hiraoka N, Okusaka T, Kubo M, Marubashi S, Yamada T, Hirano S, Yamamoto M, Ohdan H, Shimada K, Ishikawa O, Yamaue H, Chayama K, Miyano S, Aburatani H, Shibata T, Nakagawa H. Erratum: Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet, 48:700, 2016
21.Kobayashi S, Ueno M, Hara H, Irie K, Goda Y, Moriya S, Tezuka S, Tanaka M, Okusaka T, Ohkawa S, Morimoto M. Unexpected Side Effects of a High S-1 Dose: Subanalysis of a Phase III Trial Comparing Gemcitabine, S-1 and Combinatorial Treatments for Advanced Pancreatic Cancer. Oncology, 91:117-126, 2016
22.Park JO, Ryoo B-Y, Yen C-J, Kudo M, Yang L, Abada PB, Cheng R, Orlando M, Zhu AX, Okusaka T. Second-line ramucirumab therapy for advanced hepatocellular carcinoma (REACH): an East Asian and non-East Asian subgroup analysis. Oncotarget, 7:75482-75491, 2016
23.Okuma HS, Kondo S. Trends in the development of MET inhibitors for hepatocellular carcinoma. Future Oncol, 12:1275-1286, 2016
24.Shiba S, Morizane C, Hiraoka N, Sasaki M, Koga F, Sakamoto Y, Kondo S, Ueno H, Ikeda M, Yamada T, Shimada K, Kosuge T, Okusaka T. Pancreatic neuroendocrine tumors: A single-center 20-year experience with 100 patients. Pancreatology, 16:99-105, 2016