HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Pediatric Oncology
Chitose Ogawa, Tadashi Kumamoto, Yuki Aoki, Ayumu Arakawa, Tomoko Sonoda, Yasuhiro Fujiwara
Introduction
Pediatric oncology includes a wide variety of malignancies in children and adolescents such as acute leukemias and malignant lymphomas, as well as solid tumors including osteosarcoma, soft tissue sarcomas, neuroblastoma, liver tumors and retinoblastoma. Many diseases are usually chemo-sensitive and curable with appropriate treatments. The common approach to these diseases is a "risk-adapted therapy" strategy considering long-term life expectancy.
Our team and what we do
In the Department of Pediatric Oncology, patients with pediatric malignancies are managed by four pediatric oncologists. Although pediatric oncologists mainly treat and manage patients, a multidisciplinary team approach including pediatric surgeons, radiation oncologists, orthopedic surgeons, ophthalmologic surgeons and others is incorporated for the treatment. To achieve treatment completion and optimal quality of hospital life for children, pediatric nurse specialists, teachers, child care staff, psychologists and psychiatrists also join our team. For young patients, educational opportunities ranging from elementary school to high school are available in the pediatric ward where seven teachers work daily.
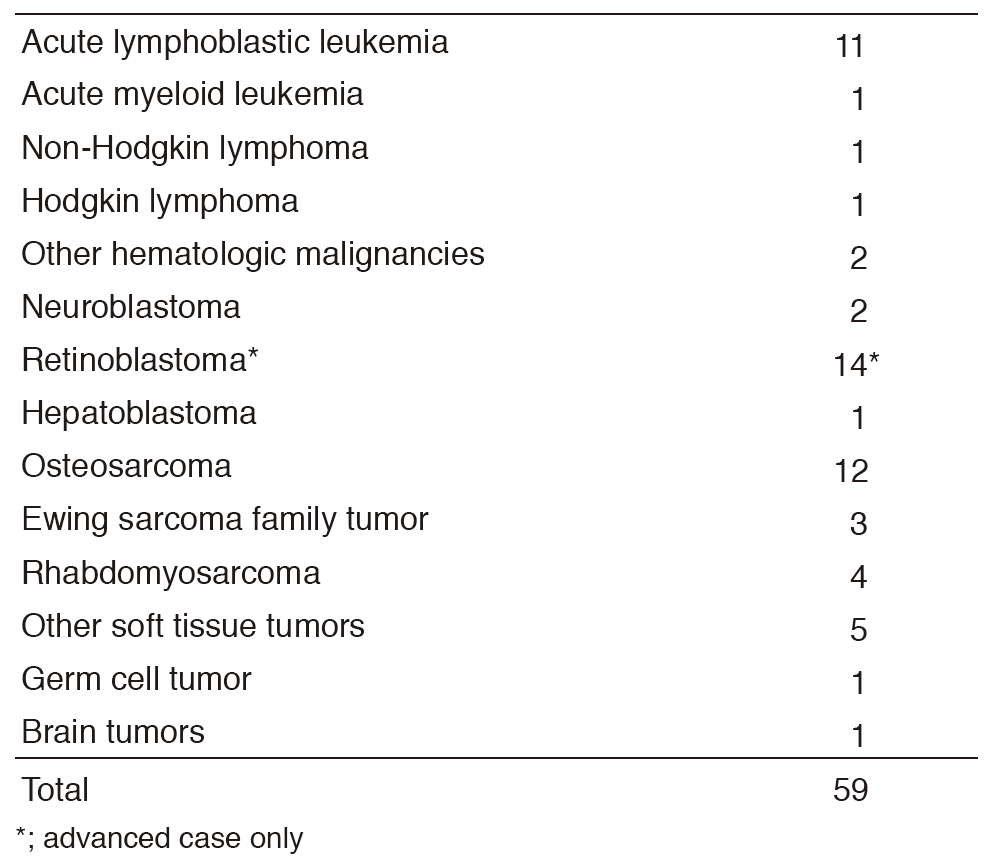
We deal with 50-70 new patients every year. Our daily activity in the pediatric outpatient clinic is to manage new patients, to treat patients with chemotherapy or blood transfusions, and to provide follow-up care for patients who have completed intensive treatment. Patients, depending on their clinical courses, receive multidisciplinary therapy, including surgical removal of the tumor, radiation therapy, chemotherapy, and sometimes stem cell transplantation (SCT).
A Pediatric Conference is held every morning, mainly to decide on individual treatment plans. The pediatric staff and trainees discuss various issues regarding pediatric inpatients on daily rounds. Inter-department conferences in cooperation with orthopedics, radiation oncology, and palliative care are individually scheduled every two weeks.
Research activities
1)For newly diagnosed patients, we participate in several multicenter studies in the Japan Children's Cancer Group (JCCG), including those by the Japan Ewing Sarcoma Study Group (JESS), the Japan Rhabdomyosarcoma Study Group (JRSG), and the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG). In addition, we also conduct our own clinical trials.
2)For relapsed patients, we are actively involved in the development of new drugs and treatments including off-label and unapproved medications.
3)For approval of new drugs, we conduct investigator-initiated registration-directed clinical trials under the Pharmaceutical Affairs Law in Japan.
4)For provision of the similar environment during the treatment for that of before patients'disease onset, we plan to construct a medical care system through the use of appropriate medical and social resources in their local communities.
Clinical trials
In 2016, we conducted 8 trials, including early phase trials, international studies, and cooperative studies. The four trials (1, 2, 7 and 8) are investigator-initiated registration-directed clinical trials. Two international cooperative trials are ongoing; in trial No.5, we are collaborating with the International BFM group in Europe, and in No. 7 with the Children's Oncology Group in the USA.
1)FMU-DF-002: Efficacy and safety study of defibrotide for treatment of veno-occlusive disease (VOD).
2)FMU-DF-003: Efficacy and safety study of defibrotide for prophylaxis of VOD.
3)JPLSG-ALL-T11 and Japan Adult Leukemia Study Group (JALSG) T-ALL-211-U ALL-T11: A Multi- Center Phase II Study in Children and Adolescence with Newly Diagnosed T-cell Acute Lymphoblastic Leukemia
4)JPLSG- ALL-B12: A Multi-Center Phase II/III Study in Children with Newly Diagnosed B-cell Precursor Acute Lymphoblastic Leukemia
5)An International Study for Treatment of Standard Risk Childhood Relapsed ALL 2010 (IntReALL SR 2010): A randomized Phase III Study Conducted by the Resistant Disease Committee of the International BFM Study Group
6)JPLSG-AML-12: A Multi-Center Seamless Phase II-III Randomized Trial of High-dose Cytarabine in Initial Induction with Evaluation of Flow-cytometry-based Minimal Residual Disease for Children with de Novo Acute Myeloid Leukemia
7)AHEP0731: Treatment of Children with All Stages of Hepatoblastoma with Temsirolimus Added to High Risk Stratum Treatment: A Phase III Study
8)BZM-ALL-2: A Phase II study of combination chemotherapy including Bortezomib in pediatric patients with relapsed acute lymphoblastic leukemia
Education
We provide personnel training and education for skills of diagnosis and management for all pediatric malignancies. Residents also learn the skills to treat not only newly diagnosed patients, but also relapsed or refractory patients by the global standard therapy. In addition, senior residents acquire abilities to plan studies for new agents or therapies, which we regard as an important role of our center.
Future prospects
We promote the development of therapies for pediatric malignancies as a top priority. For this mission, we lead the plan for clinical or registration trials in cooperation with domestic and international centers as a core institution in Japan. Our other mission is to provide individualized medicine for children with cancer. Based on the comprehensive genetic testing, we promote clinical trials using molecular targeted agents for pediatric malignancies.
List of papers published in 2016
Journal
1.Saito Y, Kumamoto T, Makino Y, Tamai I, Ogawa C, Terakado H. A retrospective study of treatment and prophylaxis of ifosfamide-induced hemorrhagic cystitis in pediatric and adolescent and young adult (AYA) patients with solid tumors. Jpn J Clin Oncol, 46:856-861, 2016
2.Ohara Y, Ohto H, Tasaki T, Sano H, Mochizuki K, Akaihata M, Kobayashi S, Waragai T, Ito M, Hosoya M, Nollet KE, Ikeda K, Ogawa C, Kanno T, Shikama Y, Kikuta A. Comprehensive technical and patient-care optimization in the management of pediatric apheresis for peripheral blood stem cell harvesting. Transfus Apher Sci, 55:338-343, 2016
3.Umemura K, Iwaki T, Kimura T, Ogawa C, Fukuda T, Taniguchi S, Horibe K, Goto H, Yoshimura K, Watanabe Y, Nitani C, Kikuta A. Pharmacokinetics and Safety of Defibrotide in Healthy Japanese Subjects. Clin Pharmacol Drug Dev, 5:548-551, 2016
4.Yoshihara H, Kamiya T, Hosoya Y, Hasegawa D, Ogawa C, Asanuma H, Mizuno R, Hosoya R, Manabe A. Ewing sarcoma/primitive neuroectodermal tumor of the kidney treated with chemotherapy including ifosfamide. Pediatr Int, 58:766-769, 2016
5.Koh K, Ogawa C, Okamoto Y, Kudo K, Inagaki J, Morimoto T, Mizukami H, Ecstein-Fraisse E, Kikuta A. Phase 1 study of clofarabine in pediatric patients with relapsed/refractory acute lymphoblastic leukemia in Japan. Int J Hematol, 104:245-255, 2016
6.Endo A, Tomizawa D, Aoki Y, Morio T, Mizutani S, Takagi M. EWSR1/ELF5 induces acute myeloid leukemia by inhibiting p53/p21 pathway. Cancer Sci, 107:1745-1754, 2016