HOME > Publication & Reports > Annual Report 2016 > Hospital
Department of Experimental Therapeutics
Noboru Yamamoto, Toshio Shimizu, Yutaka Fujiwara, Kan Yonemori, Kiyoshi Yoshimura, Shigehisa Kitano, Shunsuke Kondo, Satoru Iwasa, Akihiko Shimomura, Takafumi Koyama
Introduction
In April 2015, the affiliation of the Department of Experimental Therapeutics was changed from the National Cancer Center (NCC) - the Exploratory Oncology Research & Clinical Trial Center (EPOC) to the NCC-Hospital. The goal of our department is to perform initial clinical evaluation of promising new anti-cancer compounds emerging from the laboratory in phase I trials. The staff of this department consists of specialists from various oncology fields (i.e., thoracic oncology, breast & medical oncology, gastro-intestinal oncology, hepato-biliary & pancreatic oncology, and immuno-oncology).
Our team and what we do
This department plays a key role of the new anti-cancer drug development in Japan, as well as in Asia. The top priority is to conduct the FIH trials, and is to also perform the phase I trials for solid tumors (i.e., all comers). Recently, we have been joining the global phase I trials to accelerate the new drug development in Japan. Web- or tel- conferences are held with the EU and US sites, and we discuss patient enrollment as well as further developmental strategy. Routine web- conferences are also held between the NCC-Hospital (Tokyo) and the NCC-Hospital East (Chiba) every Friday morning, and we share information about adverse events, patient enrollment, and refer candidates to each other in order to accelerate enrollment. Nowadays, most of the phase I trials (i.e., first in Japanese phase I trial, first in human trial) in Japan are conducted at the NCC-Hospital and the NCC-Hospital East in close collaboration.
Research activities
The elucidation of the proof of concept is essential in the new anti-cancer drug development especially in the early phase, so we conduct several translational researches (TR) in collaboration with the adjoining NCC-Research Institute. Comprehensive genomic analyses, named TOP-GEAR-study (UMIN000011141), are ongoing to facilitate the patient enrollment for new molecular targeted drugs under investigation. Also, we are conducting the TR with the pharmaceutical industry to discover new targets for anti-immune therapy using human tissue (tumor and normal tissue) samples.
Clinical trials
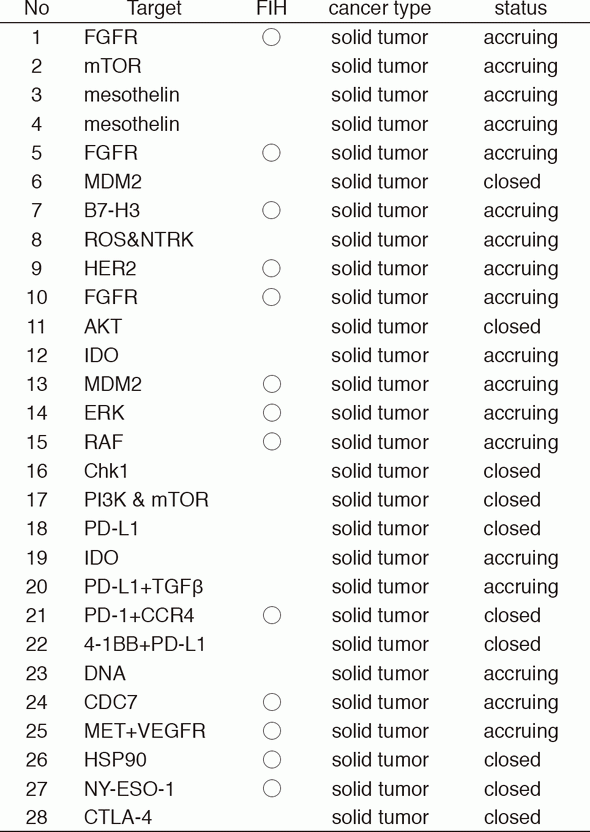
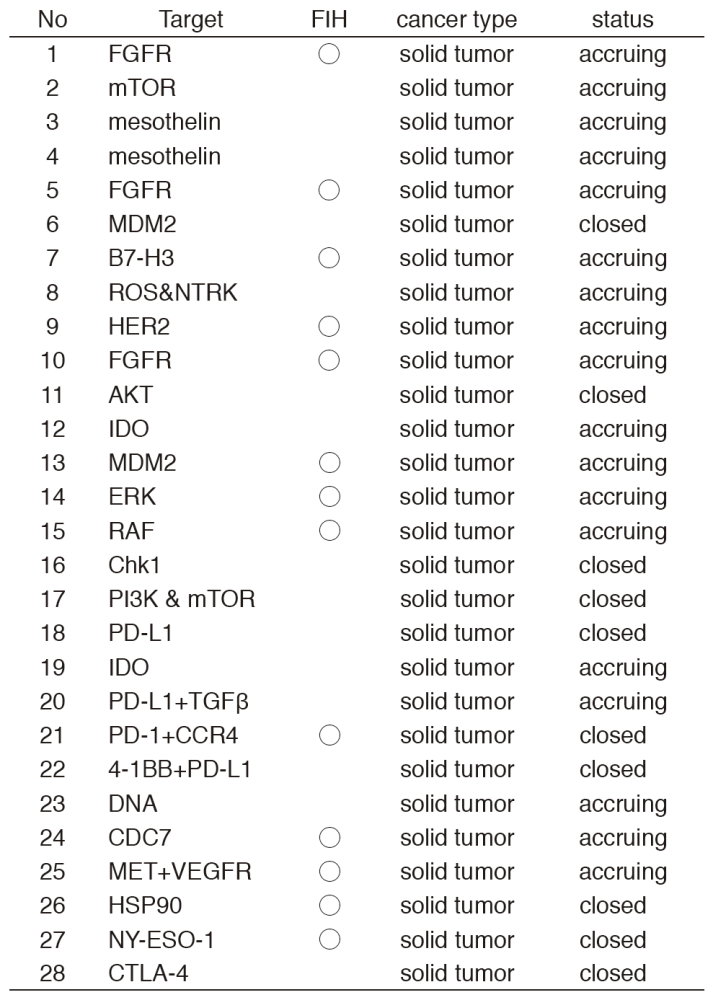
In 2016, 28 phase I trials including 13 FIH trials were conducted (Table 1).
Education
In 2016, 1st NCCH Workshop on Methods in Oncology Phase I trials and Translational Research was held on 15-OCT-2016 at the NCC-Tsukiji campus.
Table 1. Phase I trials conducted in 2016
List of papers published in 2016
Journal
1.Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Okuyama H, Ueno H, Morizane C, Kondo S, Sakamoto Y, Okusaka T, Ochiai A. C-Reactive Protein Level Is an Indicator of the Aggressiveness of Advanced Pancreatic Cancer. Pancreas, 45:110-116, 2016
2.Harano K, Yonemori K, Hirakawa A, Shimizu C, Katsumata N, Gemma A, Fujiwara Y, Tamura K. The influence of familial factors on the choice of the place of death for terminally ill breast cancer patients: a retrospective single-center study. Breast Cancer, 23:797-806, 2016
3.Nokihara H, Yamada Y, Fujiwara Y, Yamamoto N, Wakui H, Nakamichi S, Kitazono S, Inoue K, Harada A, Taube T, Takeuchi Y, Tamura T. Phase I trial of volasertib, a Polo-like kinase inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs, 34:66-74, 2016
4.Kurihara H, Shimizu C, Miyakita Y, Yoshida M, Hamada A, Kanayama Y, Yonemori K, Hashimoto J, Tani H, Kodaira M, Yunokawa M, Yamamoto H, Watanabe Y, Fujiwara Y, Tamura K. Molecular imaging using PET for breast cancer. Breast Cancer, 23:24-32, 2016
5.Shimizu C, Mogushi K, Morioka MS, Yamamoto H, Tamura K, Fujiwara Y, Tanaka H. Fc-Gamma receptor polymorphism and gene expression of peripheral blood mononuclear cells in patients with HER2-positive metastatic breast cancer receiving single-agent trastuzumab. Breast Cancer, 23:624-632, 2016
6.Shimomura A, Fujiwara Y, Kondo S, Kodaira M, Iwasa S, Kitano S, Tanabe Y, Tamura K, Yamamoto N. Tremelimumab-associated tumor regression following after initial progression: two case reports. Immunotherapy, 8:9-15, 2016
7.Shoji H, Morizane C, Sakamoto Y, Kondo S, Ueno H, Takahashi H, Ohno I, Shimizu S, Mitsunaga S, Ikeda M, Okusaka T. Phase I clinical trial of oral administration of S-1 in combination with intravenous gemcitabine and cisplatin in patients with advanced biliary tract cancer. Jpn J Clin Oncol, 46:132-137, 2016
8.Yamamoto S, Suga K, Maeda K, Maeda N, Yoshimura K, Oka M. Breast sentinel lymph node navigation with three-dimensional computed tomography-lymphography: a 12-year study. Breast Cancer, 23:456-462, 2016
9.Yamashita M, Kitano S, Aikawa H, Kuchiba A, Hayashi M, Yamamoto N, Tamura K, Hamada A. A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells. Sci Rep, 6:19772, 2016
10.Seki Y, Fujiwara Y, Kohno T, Takai E, Sunami K, Goto Y, Horinouchi H, Kanda S, Nokihara H, Watanabe S, Ichikawa H, Yamamoto N, Kuwano K, Ohe Y. Picoliter-Droplet Digital Polymerase Chain Reaction-Based Analysis of Cell-Free Plasma DNA to Assess EGFR Mutations in Lung Adenocarcinoma That Confer Resistance to Tyrosine-Kinase Inhibitors. Oncologist, 21:156-164, 2016
11.Takahashi N, Furuta K, Taniguchi H, Sasaki Y, Shoji H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Hamaguchi T, Shimada Y, Yamada Y. Serum level of hepatocyte growth factor is a novel marker of predicting the outcome and resistance to the treatment with trastuzumab in HER2-positive patients with metastatic gastric cancer. Oncotarget, 7:4925-4938, 2016
12.Araki T, Takashima A, Hamaguchi T, Honma Y, Iwasa S, Okita N, Kato K, Yamada Y, Hashimoto H, Taniguchi H, Kushima R, Nakao K, Boku N, Shimada Y. Amrubicin in patients with platinum-refractory metastatic neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the gastrointestinal tract. Anticancer Drugs, 27:794-799, 2016
13.Bun S, Yunokawa M, Ebata T, Shimomura A, Shimoi T, Kodaira M, Yonemori K, Shimizu C, Fujiwara Y, Kato T, Makino Y, Hayashi Y, Tamura K. Feasibility of dose-dense paclitaxel/carboplatin therapy in elderly patients with ovarian, fallopian tube, or peritoneal cancer. Cancer Chemother Pharmacol, 78:745-752, 2016
14.Ebata T, Yunokawa M, Bun S, Shimomura A, Shimoi T, Kodaira M, Yonemori K, Shimizu C, Fujiwara Y, Kato T, Tamura K. Dose-dense paclitaxel plus carboplatin as neoadjuvant chemotherapy for advanced ovarian, fallopian tube, or primary peritoneal carcinomas. Cancer Chemother Pharmacol, 78:1283-1288, 2016
15.Fujiwara K, Shintani D, Nishikawa T. Clear-cell carcinoma of the ovary. Ann Oncol, 27 Suppl 1:i50-i52, 2016
16.Fujiwara Y, Hamada A, Mizugaki H, Aikawa H, Hata T, Horinouchi H, Kanda S, Goto Y, Itahashi K, Nokihara H, Yamamoto N, Ohe Y. Pharmacokinetic profiles of significant adverse events with crizotinib in Japanese patients with ABCB1 polymorphism. Cancer Sci, 107:1117-1123, 2016
17.Fujiwara Y, Tamura K, Kondo S, Tanabe Y, Iwasa S, Shimomura A, Kitano S, Ogasawara K, Turner PK, Mori J, Asou H, Chan EM, Yamamoto N. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol, 78:281-288, 2016
18.Funakoshi Y, Fujiwara Y, Kiyota N, Mukohara T, Shimada T, Toyoda M, Imamura Y, Chayahara N, Tomioka H, Umezu M, Otsuki N, Nibu K, Minami H. Validity of new methods to evaluate renal function in cancer patients treated with cisplatin. Cancer Chemother Pharmacol, 77:281-288, 2016
19.Harano K, Hirakawa A, Yunokawa M, Nakamura T, Satoh T, Nishikawa T, Aoki D, Ito K, Ito K, Nakanishi T, Susumu N, Takehara K, Watanabe Y, Watari H, Saito T. Optimal cytoreductive surgery in patients with advanced uterine carcinosarcoma: A multi-institutional retrospective study from the Japanese gynecologic oncology group. Gynecol Oncol, 141:447-453, 2016
20.Hatogai K, Kitano S, Fujii S, Kojima T, Daiko H, Nomura S, Yoshino T, Ohtsu A, Takiguchi Y, Doi T, Ochiai A. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget, 7:47252-47264, 2016
21.Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Sumi M, Tamura T, Ohe Y. Candidates for Intensive Local Treatment in cIIIA-N2 Non-Small Cell Lung Cancer: Deciphering the Heterogeneity. Int J Radiat Oncol Biol Phys, 94:155-162, 2016
22.Iwasa S, Yamada Y, Heike Y, Shoji H, Honma Y, Komatsu N, Matsueda S, Yamada A, Morita M, Yamaguchi R, Tanaka N, Kawahara A, Kage M, Shichijo S, Sasada T, Itoh K. Phase I study of a new cancer vaccine of ten mixed peptides for advanced cancer patients. Cancer Sci, 107:590-600, 2016
23.Kanda S, Goto K, Shiraishi H, Kubo E, Tanaka A, Utsumi H, Sunami K, Kitazono S, Mizugaki H, Horinouchi H, Fujiwara Y, Nokihara H, Yamamoto N, Hozumi H, Tamura T. Safety and efficacy of nivolumab and standard chemotherapy drug combination in patients with advanced non-small-cell lung cancer: a four arms phase Ib study. Ann Oncol, 27:2242-2250, 2016
24.Kataoka T, Kiyota N, Shimada T, Funakoshi Y, Chayahara N, Toyoda M, Fujiwara Y, Nibu K, Komori T, Sasaki R, Mukohara T, Minami H. Randomized trial of standard pain control with or without gabapentin for pain related to radiation-induced mucositis in head and neck cancer. Auris Nasus Larynx, 43:677-684, 2016
25.Katsuya Y, Horinouchi H, Asao T, Kitahara S, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Watanabe S, Tsuta K, Ohe Y. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: Impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer, 99:4-10, 2016
26.Kim Y, Kobayashi E, Kubota D, Suehara Y, Mukaihara K, Akaike K, Ito A, Kaneko K, Chuman H, Kawai A, Kitano S. Reduced argininosuccinate synthetase expression in refractory sarcomas: Impacts on therapeutic potential and drug resistance. Oncotarget, 7:70832-70844, 2016
27.Kimbara S, Kondo S. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J Gastroenterol, 22:7440-7452, 2016
28.Makino Y, Watanabe M, Makihara RA, Nokihara H, Yamamoto N, Ohe Y, Sugiyama E, Sato H, Hayashi Y. Simultaneous optimization of limited sampling points for pharmacokinetic analysis of amrubicin and amrubicinol in cancer patients. Asia Pac J Clin Oncol, 12:259-264, 2016
29.Matsukuma S, Yoshimura K, Ueno T, Oga A, Inoue M, Watanabe Y, Kuramasu A, Fuse M, Tsunedomi R, Nagaoka S, Eguchi H, Matsui H, Shindo Y, Maeda N, Tokuhisa Y, Kawano R, Furuya-Kondo T, Itoh H, Yoshino S, Hazama S, Oka M, Nagano H. Calreticulin is highly expressed in pancreatic cancer stem-like cells. Cancer Sci, 107:1599-1609, 2016
30.Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, Sheridan TB, Bush SH, Klobocista MM, Blake EA, Takano T, Matsuzaki S, Baba T, Satoh S, Shida M, Nishikawa T, Ikeda Y, Adachi S, Yokoyama T, Takekuma M, Fujiwara K, Hazama Y, Kadogami D, Moffitt MN, Takeuchi S, Nishimura M, Iwasaki K, Ushioda N, Johnson MS, Yoshida M, Hakam A, Li SW, Richmond AM, Machida H, Mhawech-Fauceglia P, Ueda Y, Yoshino K, Yamaguchi K, Oishi T, Kajiwara H, Hasegawa K, Yasuda M, Kawana K, Suda K, Miyake TM, Moriya T, Yuba Y, Morgan T, Fukagawa T, Wakatsuki A, Sugiyama T, Pejovic T, Nagano T, Shimoya K, Andoh M, Shiki Y, Enomoto T, Sasaki T, Mikami M, Shimada M, Konishi I, Kimura T, Post MD, Shahzad MM, Im DD, Yoshida H, Omatsu K, Ueland FR, Kelley JL, Karabakhtsian RG, Roman LD. Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann Oncol, 27:1257-1266, 2016
31.Minami H, Ando Y, Ma BBY, Hsiang Lee J, Momota H, Fujiwara Y, Li L, Fukino K, Ito K, Tajima T, Mori A, Lin C-C. Phase I, multicenter, open-label, dose-escalation study of sonidegib in Asian patients with advanced solid tumors. Cancer Sci, 107:1477-1483, 2016
32.Miyasaka A, Nishikawa T, Kozawa E, Yasuda M, Fujiwara K, Hasegawa K. Advanced Mucinous Adenocarcinoma Arising from a Mature Cystic Teratoma: A Case Report and Literature Review. Case Rep Oncol, 9:331-337, 2016
33.Asao T, Fujiwara Y, Sunami K, Kitahara S, Goto Y, Kanda S, Horinouchi H, Nokihara H, Yamamoto N, Ichikawa H, Kohno T, Tsuta K, Watanabe S, Takahashi K, Ohe Y. Medical treatment involving investigational drugs and genetic profile of thymic carcinoma. Lung Cancer, 93:77-81, 2016
34.Mizugaki H, Yamamoto N, Murakami H, Kenmotsu H, Fujiwara Y, Ishida Y, Kawakami T, Takahashi T. Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumors. Invest New Drugs, 34:596-603, 2016
35.Nagao S, Iwasa N, Kurosaki A, Nishikawa T, Hanaoka T, Hasegawa K, Fujiwara K. The Efficacy of Low-Dose Paclitaxel Added to Combination Chemotherapy of Carboplatin and Gemcitabine or Pegylated Liposomal Doxorubicin. Int J Gynecol Cancer, 26:443-448, 2016
36.Nakamura Y, Kitano S, Takahashi A, Tsutsumida A, Namikawa K, Tanese K, Abe T, Funakoshi T, Yamamoto N, Amagai M, Yamazaki N. Nivolumab for advanced melanoma: pretreatment prognostic factors and early outcome markers during therapy. Oncotarget, 7:77404-77415, 2016
37.Nokihara H, Yamamoto N, Ohe Y, Hiraoka M, Tamura T. Pharmacokinetics of Weekly Paclitaxel and Feasibility of Dexamethasone Taper in Japanese Patients with Advanced Non-small Cell Lung Cancer. Clin Ther, 38:338-347, 2016
38.Sasaki Y, Hamaguchi T, Yamada Y, Takahashi N, Shoji H, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Nagai Y, Taniguchi H, Boku N, Ushijima T, Shimada Y. Value of KRAS, BRAF, and PIK3CA Mutations and Survival Benefit from Systemic Chemotherapy in Colorectal Peritoneal Carcinomatosis. Asian Pac J Cancer Prev, 17:539-543, 2016
39.Nomura M, Iwasa S, Tsushima T, Kato K, Yasui H, Boku N, Muto M, Muro K. Active salvage chemotherapy versus best supportive care for patients with recurrent or metastatic squamous cell carcinoma of the esophagus refractory or intolerable to fluorouracil, platinum, and taxane. Cancer Chemother Pharmacol, 78:1209-1216, 2016
40.Nonagase Y, Okamoto K, Iwasa T, Yoshida T, Tanaka K, Takeda M, Kaneda H, Shimizu T, Tsurutani J, Nakagawa K. Afatinib-refractory brain metastases from EGFR-mutant non-small-cell lung cancer successfully controlled with erlotinib: a case report. Anticancer Drugs, 27:251-253, 2016
41.Ogiya R, Niikura N, Kumaki N, Bianchini G, Kitano S, Iwamoto T, Hayashi N, Yokoyama K, Oshitanai R, Terao M, Morioka T, Tsuda B, Okamura T, Saito Y, Suzuki Y, Tokuda Y. Comparison of tumor-infiltrating lymphocytes between primary and metastatic tumors in breast cancer patients. Cancer Sci, 107:1730-1735, 2016
42.Okuma HS, Koizumi F, Hirakawa A, Nakatochi M, Komori O, Hashimoto J, Kodaira M, Yunokawa M, Yamamoto H, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Clinical and microarray analysis of breast cancers of all subtypes from two prospective preoperative chemotherapy studies. Br J Cancer, 115:411-419, 2016
43.Okuma HS, Kondo S. Trends in the development of MET inhibitors for hepatocellular carcinoma. Future Oncol, 12:1275-1286, 2016
44.Sasada S, Kodaira M, Shimoi T, Shimomura A, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Ifosfamide and Etoposide Chemotherapy in the Treatment of Recurrent/Refractory Rhabdomyosarcoma in Adults. Anticancer Res, 36:2429-2432, 2016
45.Satake H, Tahara M, Mochizuki S, Kato K, Hara H, Yokota T, Kiyota N, Kii T, Chin K, Zenda S, Kojima T, Bando H, Yamazaki T, Iwasa S, Honma Y, Hamauchi S, Tsushima T, Ohtsu A. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol, 78:91-99, 2016
46.Shiba S, Morizane C, Hiraoka N, Sasaki M, Koga F, Sakamoto Y, Kondo S, Ueno H, Ikeda M, Yamada T, Shimada K, Kosuge T, Okusaka T. Pancreatic neuroendocrine tumors: A single-center 20-year experience with 100 patients. Pancreatology, 16:99-105, 2016
47.Shigeta K, Kosaka T, Kitano S, Yasumizu Y, Miyazaki Y, Mizuno R, Shinojima T, Kikuchi E, Miyajima A, Tanoguchi H, Hasegawa S, Oya M. High Absolute Monocyte Count Predicts Poor Clinical Outcome in Patients with Castration-Resistant Prostate Cancer Treated with Docetaxel Chemotherapy. Ann Surg Oncol, 23:4115-4122, 2016
48.Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M, Nakagawa K. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77:997-1003, 2016
49.Shimizu T, Seto T, Hirai F, Takenoyama M, Nosaki K, Tsurutani J, Kaneda H, Iwasa T, Kawakami H, Noguchi K, Shimamoto T, Nakagawa K. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs, 34:347-354, 2016
50.Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, Okita N, Takashima A, Kato K, Yamada Y, Katayama N, Boku N, Heike Y, Hamaguchi T. Pretreatment Immune Status Correlates with Progression-Free Survival in Chemotherapy-Treated Metastatic Colorectal Cancer Patients. Cancer Immunol Res, 4:592-599, 2016
51.Takahashi N, Iwasa S, Fukahori M, Sudo K, Sasaki Y, Shoji H, Honma Y, Okita NT, Takashima A, Hamaguchi T, Boku N, Shimada Y, Honda K, Yamada T, Yamada Y. A phase I study of the combination of panitumumab and bevacizumab in KRAS wild-type colorectal cancer patients previously treated with fluoropyrimidine, oxaliplatin, irinotecan and bevacizumab. Cancer Chemother Pharmacol, 78:567-575, 2016
52.Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol, 142:1727-1738, 2016
53.Takahashi N, Iwasa S, Taniguchi H, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita N, Kato K, Hamaguchi T, Shimada Y, Yamada Y. Prognostic role of ERBB2, MET and VEGFA expression in metastatic colorectal cancer patients treated with anti-EGFR antibodies. Br J Cancer, 114:1003-1011, 2016
54.Tamura K, Hashimoto J, Tanabe Y, Kodaira M, Yonemori K, Seto T, Hirai F, Arita S, Toyokawa G, Chen L, Yamamoto H, Kawata T, Lindemann J, Esaki T. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 77:787-795, 2016
55.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci, 107:755-763, 2016
56.Tamura Y, Nokihara H, Honda K, Tanabe Y, Asahina H, Yamada Y, Enatsu S, Kurek R, Yamamoto N, Tamura T. Phase I study of the second-generation, recombinant, human EGFR antibody necitumumab in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol, 78:995-1002, 2016
57.Tanabe Y, Ichikawa H, Kohno T, Yoshida H, Kubo T, Kato M, Iwasa S, Ochiai A, Yamamoto N, Fujiwara Y, Tamura K. Comprehensive screening of target molecules by next-generation sequencing in patients with malignant solid tumors: guiding entry into phase I clinical trials. Mol Cancer, 15:73, 2016
58.Tanaka R, Yonemori K, Hirakawa A, Kinoshita F, Takahashi N, Hashimoto J, Kodaira M, Yamamoto H, Yunokawa M, Shimizu C, Fujimoto M, Fujiwara Y, Tamura K. Risk Factors for Developing Skeletal-Related Events in Breast Cancer Patients With Bone Metastases Undergoing Treatment With Bone-Modifying Agents. Oncologist, 21:508-513, 2016
59.Watanabe S, Takeda M, Takahama T, Iwasa T, Tsurutani J, Tanizaki J, Shimizu T, Sakai K, Wada Y, Isogai N, Nishio K, Nakagawa K. Successful human epidermal growth receptor 2-targeted therapy beyond disease progression for extramammary Paget's disease. Invest New Drugs, 34:394-396, 2016
60.Yamaguchi T, Iwasa S, Nagashima K, Ikezawa N, Hamaguchi T, Shoji H, Honma Y, Takashima A, Okita N, Kato K, Yamada Y, Shimada Y. Comparison of Panitumumab Plus Irinotecan and Cetuximab Plus Irinotecan for KRAS Wild-type Metastatic Colorectal Cancer. Anticancer Res, 36:3531-3536, 2016
61.Yonemori K, Hirakawa A, Kawachi A, Kinoshita F, Okuma H, Nishikawa T, Tamura K, Fujiwara Y, Takebe N. Drug induced interstitial lung disease in oncology phase I trials. Cancer Sci, 107:1830-1836, 2016
62.Yonemori K, Tamura K, Kodaira M, Fujikawa K, Sagawa T, Esaki T, Shirakawa T, Hirai F, Yokoi Y, Kawata T, Hatano B, Takahashi Y. Safety and tolerability of the olaparib tablet formulation in Japanese patients with advanced solid tumours. Cancer Chemother Pharmacol, 78:525-531, 2016
63.Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, Ono M, Takeshita F, Niida S, Shimizu C, Fujiwara Y, Kinoshita T, Tamura K, Ochiya T. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci, 107:326-334, 2016
64.Miura N, Kamita M, Kakuya T, Fujiwara Y, Tsuta K, Shiraishi H, Takeshita F, Ochiya T, Shoji H, Huang W, Ohe Y, Yamada T, Honda K. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget, 7:33165-33178, 2016
65.Honma Y, Yamada Y, Terazawa T, Takashima A, Iwasa S, Kato K, Hamaguchi T, Shimada Y, Ohashi M, Morita S, Fukagawa T, Machida N, Katai H. Feasibility of neoadjuvant S-1 and oxaliplatin followed by surgery for resectable advanced gastric adenocarcinoma. Surg Today, 46:1076-1082, 2016
