HOME > Publication & Reports > Annual Report 2016 > Hospital
Outpatient Treatment Center
Kenji Tamura, Hiroshi Nokihara, Dai Maruyama, Hidehito Horinouchi, Shunsuke Kondo, Satoru Iwasa, Tadashi Kumamoto, Yasuji Miyakita, Tomohiko Hara, Atsuko Kitano, Miho Kurihara, Mayumi Tsukagoshi, Hiroe Ohara, Mayu Harada, Tomonobu Otsuka, Hironobu Hashimoto, Toru Akagi, Satoshi Nakajima, Koichi Ishikawa
Introduction
The Outpatient Treatment Center deals with all kinds of cancer patients who have received chemotherapies as an outpatient style. Our mission is to provide safe, comfortable and high quality chemotherapies. Several groups collaborate to ensure the best chemotherapies, consisting of medical oncologists, nurses, pharmacists, medical social workers (MSWs), and clinical research coordinators (CRCs). Our visions are as follows.
1)To provide evidence-based medicine (EBM), and to develop novel anticancer drugs.
2)To provide safe and efficient treatments, and to ensure the management of adverse events.
3)To create a comfortable environment, and to maintain the quality of life (QOL) of patients.
Our team and what we do
1.Setup
The Outpatient Treatment Center consists of one director (doctor) , ten other medical doctors, one nurse manager, two deputy nurse managers, one deputy drug director, one chief pharmacist, one dispensing chief, one chief engineer of the Clinical Laboratories, 23 nurses, three pharmacists, and two to three reception staff.
2.Performance
We established a second Outpatient Treatment Center in the beginning of 2015. There are 30 beds in the first Outpatient Treatment Center and 26 in the second Outpatient Treatment Center (total 56). We also have six beds for general infusions or blood transfusions.
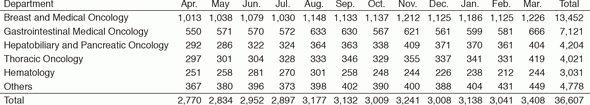
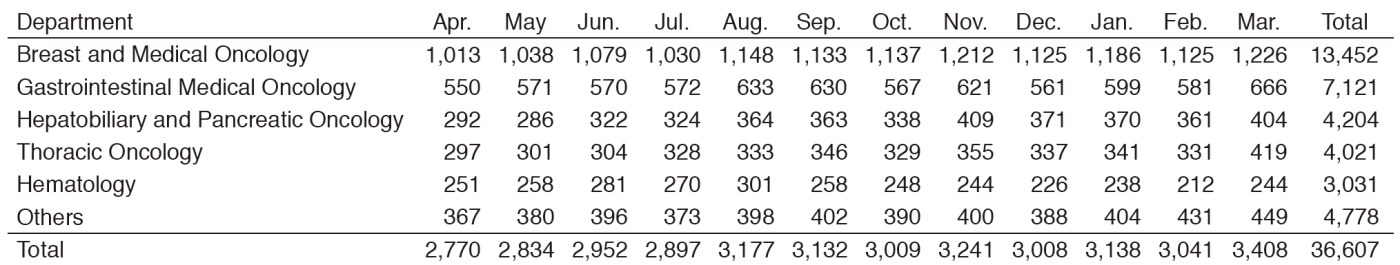
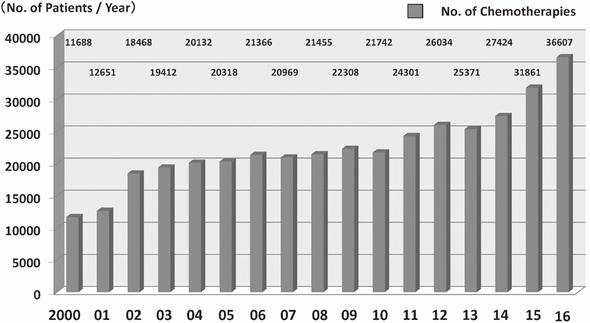
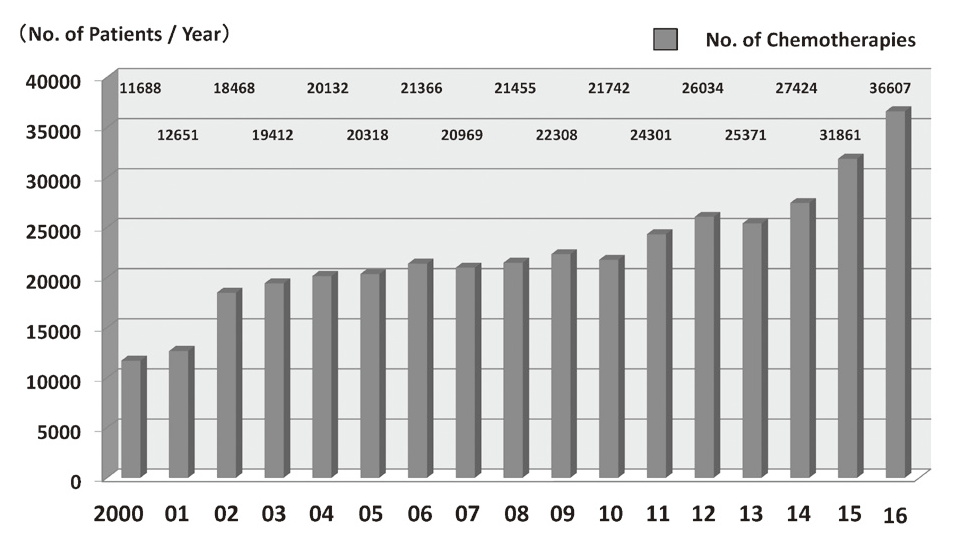
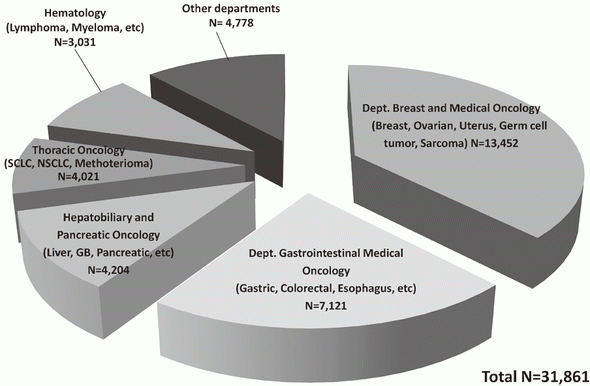
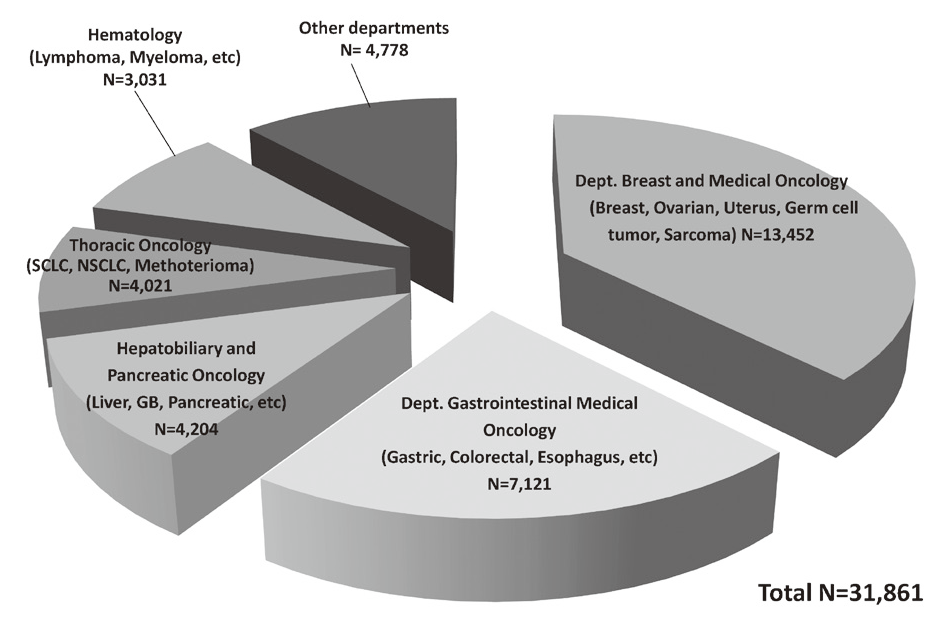
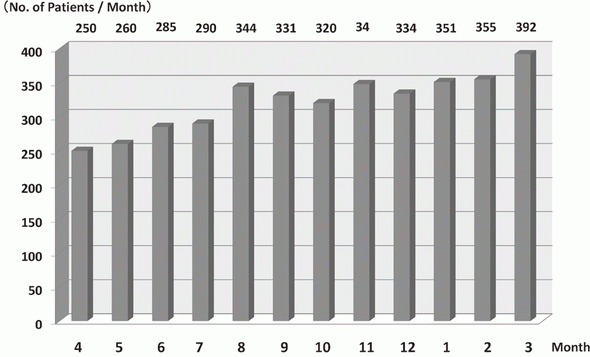
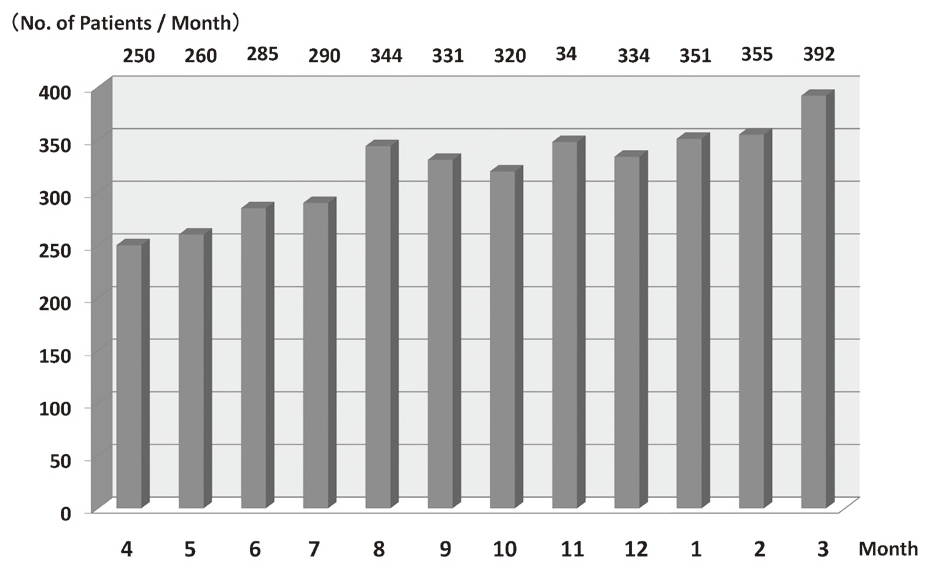
In 2016, the Outpatient Treatment Center supported 36,607 patients who received anticancer drugs (Figure 1). The breakdown by department was Breast and Medical Oncology (n=13,452), Gastrointestinal Medical Oncology (n=7,121), Hepatobiliary and Pancreatic Oncology (n=4,204), Thoracic Oncology (n=4,021), Hematology (n=3,031), and other departments (n=4,778) (Table 1, Figure 2). Clinical trials for unapproved drugs increased to around 321 cases per month (10.5% of the total cases, Figure 3). General infusions, general intramuscular or subcutaneous injections, blood transfusions, bone marrow puncture, lumbar puncture, intraperitoneal or chest drainage, and blood gas analyses were conducted in our center.
3.Staff meeting
The monthly staff meeting is held on the second Tuesday of every month at 16:30-17:30 with the participation of physicians and nurses who are main members of our center. The steering committee is held on the third Thursday of every month.
4.Hot line and conference
We conducted a telephone consultation service (Hot line) for outpatients who have received chemotherapies. We have around 100 Hot line cases per month. A Case conference, especially about the Hot line, is held monthly on a Tuesday with the participation of multidisciplinary specialists, including medical oncologists, nurses, and pharmacists.
Research activities
* Treatment of platinum-containing regime in outpatient style
* Efficacy of frozen globe against nail toxicities by docetaxel
* Protection of allergic reaction by Oxaliplatin in outpatients
* Management of skin toxicities as an adverse event of molecular-targeted drugs
* Cosmetic support for female cancer patients
* Support for continuing working for outpatients
* Telephone hot line for emergencies for outpatients who receive chemotherapies
* Monitoring adverse events of immune check point inhibitors
Education
We provide educational opportunities for multidisciplinary specialists, including medical oncologists, nurses, and pharmacists. We also provide an educational program directed outside the hospital for medical oncologists, nurses, pharmacists, and MSWs in specially designed hospitals for cancer treatment in each prefecture.
Future prospects
We are planning to undertake more activities in the second Outpatient Treatment Center, and continue to propose a model for more clinical trials in an outpatient style. We aim to shorten waiting times, undertake the smooth administration of novel molecular targeted drugs for outpatients, put into practice multidisciplinary care, and create a comfortable environment for cancer patients who received chemotherapies in the Outpatient Treatment Center.
Table 1. Cumulative total number of patients who received anticancer drugs by intravenous administration (2016)