HOME > Publication & Reports > Annual Report 2016 > Hospital
Cancer Screening Center
Takahisa Matsuda, Masau Sekiguchi, Gen Iinuma, Nachiko Uchiyama, Hiroaki Kurihara, Miyuki Sone, Yasunori Mizuguchi, Hirokazu Watanabe, Mari Kikuchi, Mototaka Miyake, Syunsuke Sugawara, Yuko Kubo, Takahiro Morita, Koji Tomita, Shinji Wada, Yasuaki Arai, Yasuo Kakugawa, Minori Matsumoto, Masayoshi Yamada, Hiroyuki Takamaru, Takaaki Tsuchida, Tomoyasu Kato, Shunichi Ikeda, Mitsuya Ishikawa, Takashi Uehara, Hanako Shimizu, Hiroshi Saito
Introduction
In the Cancer Screening Center (former name: The Research Center for Cancer Prevention and Screening: RCCPS), we provide opportunistic cancer screening widely by using newly developed modalities since 2004. Our Center consists of 14 radiologists, six gastroenterologists, one bronchoscopist, five gynecologists, seven radiologic technologists, four ultrasonographic technologists, and four nurses. Most of the staff doctors hold concurrent positions in their own specialized departments. We are in charge of multiphasic cancer screening using several imaging modalities to develop new cancer screening systems and to assess new screening tests. All medical images are digitalized and all imaging diagnosis can be made from CRT monitors.
Our Team and What we do
1.Course of cancer screening
Basic plan for males consists of screening for cancer of the lung, esophagus, stomach, colorectum, liver, gall bladder, pancreas, kidney, and prostate. In the basic plan for females, the screening for cancers of the breast, uterus, and ovary are added to the plan for males, excluding the prostate. In addition, Positron Emission Tomography (PET) is provided as an option. Other than multiphasic programs, independent cancer screening program has been prepared for lung and female genital cancers, including cancers of the uterus and ovary, breast cancer, and gastrointestinal cancer. Blood samples are also obtained for biochemistry and tumor markers such as CA19-9, CEA, CA125, PSA, and genetic analysis.
2.Eligibility criteria for participants
The cancer screening program at the Cancer Screening Center (former name: RCCPS) before 2013 has been planned for applicants aged 40 years or older who give written informed consent for the screening, including blood samples for genetic analysis, and who take the questionnaire survey concerning lifestyles. These study protocols have been approved by the Institutional Review Board (IRB). Applicants who have been diagnosed as having cancer, and/or have a history of cancer treatment, such as surgery or endoscopic mucosal resection or chemotherapy within the previous one year, are excluded. In contrast, there is no condition setting to receive cancer screening programs about the new participants after May 2014. But an inclusion agreement about the study is optionally demanded.
3.Cancer screening methods
In the multiphasic cancer screening programs, computed tomography (CT) for lung cancer, abdominal ultrasound (US) for cancer of the liver, gall bladder, pancreas, and kidney, gynecological examinations with pap-smear and human papillomavirus (HPV) test for uterus cancer, and Mammography (MMG) /Tomosynthesis and US for breast cancer are performed on the first day. On the following day, gastroscopy for cancer of the esophagus and stomach, and total colonoscopy for cancer of the colon and rectum are conducted. If a barium enema is chosen, the examination is carried out on the third day. Moreover, since the beginning of December 2010, CT-colonography (CTC) has been provided as an optional method for cancer screening. Fluorodexyglucose (FDG)-PET is offered on the first day as an option, if the participants wish to undergo the examination. In addition, the one day cancer screening program with the combination of gastrointestinal endoscopic examinations and other methods except PET, or the combination of PET and other methods excepting total colonoscopy were newly started in May 2014. Furthermore, methionin PET-CT/Magnetic Resonance Imaging (MRI) has been provided as an optional examination since 2016.
4.Number of participants of cancer screening
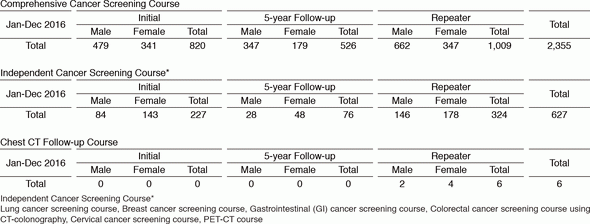
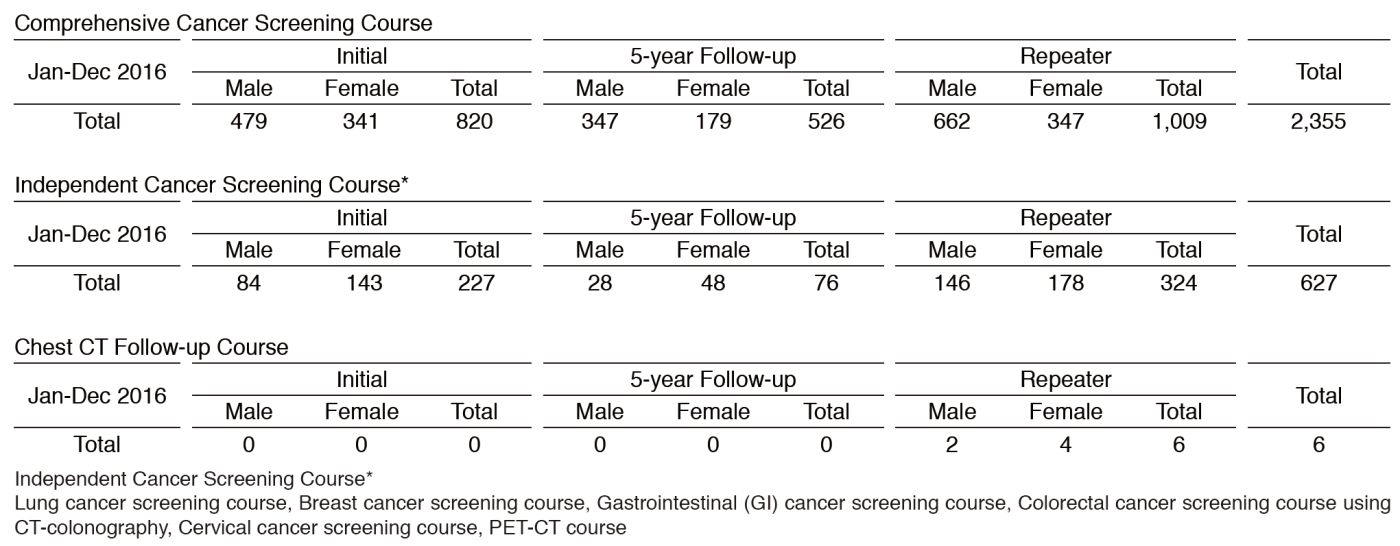
We present the number of participants of cancer screening during the period from January to December 2016 in this report (Table 1). A total of 2,988 people including 1,047 initial cases received cancer screening at the Cancer Screening Center in 2016. Most of the participants (79%; n=2,355) chose the comprehensive cancer screening course. Regarding the data of cancer detection rate in each modality, we will report them in the near future.
Research activities
1)The follow-up system of pulmonary solitary solid nodules for evaluation of growth is being developed and published.
2)A large-scale analysis of diagnostic sensitivity of PET-CT for colorectal advanced neoplasm has been published.
3)In order to establish guidelines for the management of pulmonary nodules detected with low-dose chest CT screening, patients with pulmonary nodules measuring between 5 and 10 mm in diameter are being examined in the follow-up clinic.
Future prospect
Based on cancer screening data such as examination results, medical institution findings, follow-up findings, and the questionnaire survey concerning lifestyles for 10 years, we commenced to assess them supported by the National Cancer Center Research and Development Fund.
List of papers published in 2016
Journal
1.Sekiguchi M, Igarashi A, Matsuda T, Matsumoto M, Sakamoto T, Nakajima T, Kakugawa Y, Yamamoto S, Saito H, Saito Y. Optimal use of colonoscopy and fecal immunochemical test for population-based colorectal cancer screening: a cost-effectiveness analysis using Japanese data. Jpn J Clin Oncol, 46:116-125, 2016
2.Chiu H-M, Ching JYL, Wu KC, Rerknimitr R, Li J, Wu D-C, Goh KL, Matsuda T, Kim H-S, Leong R, Yeoh KG, Chong VH, Sollano JD, Ahmed F, Menon J, Sung JJY. A Risk-Scoring System Combined With a Fecal Immunochemical Test Is Effective in Screening High-Risk Subjects for Early Colonoscopy to Detect Advanced Colorectal Neoplasms. Gastroenterology, 150:617-625 e613, 2016
3.Matsumoto M, Nakajima T, Kakugawa Y, Sakamoto T, Kuribayashi S, Otake Y, Matsuda T, Kanemitsu Y, Taniguchi H, Saito Y. Surveillance using capsule endoscopy is safe in post-colectomy patients with familial adenomatous polyposis: a prospective Japanese study. Fam Cancer, 15:75-83, 2016
4.Pioche M, Matsumoto M, Takamaru H, Sakamoto T, Nakajima T, Matsuda T, Abe S, Kakugawa Y, Otake Y, Saito Y. Endocuff-assisted colonoscopy increases polyp detection rate: a simulated randomized study involving an anatomic colorectal model and 32 international endoscopists. Surg Endosc, 30:288-295, 2016
5.Sano Y, Tanaka S, Kudo S-E, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu K-I, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano H-O, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc, 28:526-533, 2016
6.Matsuda T, Oka S, Ikematsu H, Matsushita H-o, Mori Y, Takeuchi Y, Tamai N, Kawamura T, Chino A, Keum B, Khomvilai S, Uraoka T. Endoscopic diagnosis of colorectal serrated lesions: Current status and future perspectives based on the results of a questionnaire survey. Dig Endosc, 28 Suppl 1:35-42, 2016
7.Inoki K, Nakajima T, Sekine S, Sugano K, Tsukamoto S, Yamada M, Mutoh M, Sakamoto T, Matsuda T, Sekiguchi M, Ushiama M, Yoshida T, Sakamoto H, Kanemitsu Y, Saito Y. Depressed-type submucosal invasive colorectal cancer in a patient with Lynch syndrome diagnosed using short-interval colonoscopy. Dig Endosc, 28:749-754, 2016
8.Sekiguchi M, Matsuda T, Saito Y. Surveillance after endoscopic and surgical resection of colorectal cancer. Best Pract Res Clin Gastroenterol, 30:959-970, 2016
9.Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer, 19:198-205, 2016
10.Tsuruki ES, Saito Y, Abe S, Takamaru H, Yamada M, Sakamoto T, Nakajima T, Matsuda T, Sekine S, Taniguchi H. Evaluating the efficacy and safety of a novel endoscopic fluorescence imaging modality using oral 5-aminolevulinic acid for colorectal tumors. Endosc Int Open, 4:E30-35, 2016
11.Yamada M, Saito Y, Sakamoto T, Nakajima T, Kushima R, Parra-Blanco A, Matsuda T. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy, 48:456-464, 2016
12.Perez-Cuadrado Robles E, Yamada M, Saito Y. Successful balloon overtube-guided colorectal endoscopic submucosal dissection by a gastroscope. Rev Esp Enferm Dig, 108:280-281, 2016
13.Mori Y, Kudo S-e, Ogawa Y, Wakamura K, Kudo T, Misawa M, Hayashi T, Katagiri A, Miyachi H, Inoue H, Oka S, Matsuda T. Diagnosis of sessile serrated adenomas/polyps using endocytoscopy (with videos). Dig Endosc, 28 Suppl 1:43-48, 2016
14.Matsuda T, Chiu H-M, Sano Y, Fujii T, Ono A, Saito Y. Surveillance colonoscopy after endoscopic treatment for colorectal neoplasia: From the standpoint of the Asia-Pacific region. Dig Endosc, 28:342-347, 2016
15.De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol, 104:138-155, 2016
16.Takamaru H, Saito Y, Yamada M, Tsuruki ES, Kinjo Y, Otake Y, Sakamoto T, Nakajima T, Matsuda T. Clinical impact of endoscopic clip closure of perforations during endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc, 84:494-502 e491, 2016
17.Inoki K, Sakamoto T, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Saito Y. Successful endoscopic closure of a colonic perforation one day after endoscopic mucosal resection of a lesion in the transverse colon. World J Clin Cases, 4:238-242, 2016
18.Wong MCS, Ching JYL, Chiu H-M, Wu KC, Rerknimitr R, Li J, Wu D-C, Goh KL, Matsuda T, Kim H-S, Leong R, Yeoh KG, Chong VH, Sollano JD, Ahmed F, Menon J, Ng SC, Wu JCY, Chan FKL, Sung JJY. Risk of Colorectal Neoplasia in Individuals With Self-Reported Family History: A Prospective Colonoscopy Study from 16 Asia-Pacific Regions. Am J Gastroenterol, 111:1621-1629, 2016
19.Abe S, Sakamoto T, Takamaru H, Yamada M, Nakajima T, Matsuda T, Saito Y. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc, 30:5459-5464, 2016
20.Sekiguchi M, Kakugawa Y, Terauchi T, Matsumoto M, Saito H, Muramatsu Y, Saito Y, Matsuda T. Sensitivity of 2-[18F]fluoro-2-deoxyglucose positron emission tomography for advanced colorectal neoplasms: a large-scale analysis of 7505 asymptomatic screening individuals. J Gastroenterol, 51:1122-1132, 2016
21.Sekiguchi M, Oda I, Taniguchi H, Suzuki H, Morita S, Fukagawa T, Sekine S, Kushima R, Katai H. Risk stratification and predictive risk-scoring model for lymph node metastasis in early gastric cancer. J Gastroenterol, 51:961-970, 2016
22.Suzuki H, Oda I, Sekiguchi M, Abe S, Nonaka S, Yoshinaga S, Saito Y. Factors associated with incomplete gastric endoscopic submucosal dissection due to misdiagnosis. Endosc Int Open, 4:E788-793, 2016
23.Takamaru H, Yamada M, Sakamoto T, Nakajima T, Saito Y, Kakugawa Y, Matsumoto M, Matsuda T, Ide D, Saito S, Gulati S, Tajiri H. Dual camera colon capsule endoscopy increases detection of colorectal lesions. Scand J Gastroenterol, 51:1532-1533, 2016