HOME > Publication & Reports > Annual Report 2016 > Hospital
Clinical Laboratories
Satoshi Nakajima, Shigeyuki Hasuo, Masahiro Uchikawa, Susumu Wakai, Naoshi Sasaki, Motoi Miyakoshi, Koji Ono, Hiroshi Yamakawa, Ryuzaburo Otake, Youji Hashimoto, Yasuo Shibuki, Hiroki Kakishima, Tomohiro Nakatani, Michi Shohji, Tsutomu Watanabe, Rie Matsuo, Yukie Nakajima, Sachiko Kobayashi, Kazuya Tokita, Miyaki Satoe, Kumiko Nagasaki, Noriko Takahashi, Mizuho Fujima, Tomoe Ito, Midori Aikawa, Kaori Ueki, Fumie Watanabe, Akino Chiba, Takako Takada, Kyoko Osanai, Yuko Adegawa, Ruriko Miyake, Asuka Matsunaga, Hiroshi Chigira, Go Sato, Sakiko Yoshimura, Yu Aruga, Saori Kobayashi, Kaori Yamaguchi, Ryoko Uegaki, Kensyo Kashiwaya, Saori Nakabayashi, Shingo Nakajima, Hideya Matsubayashi, Saeko Shirahama, Akiko Takayanagi, Mei Fukuhara, Kumi Ohashi, Momoko Kito, Moemi Kasane, Kazuhiro Yoshida, Haruki Sasaki, Keiko Arai, Yuri Kurosawa, Hitomi Tsuchiya, Madoka Kondo, Mai Okayama, Mayu Takeno, Daiki Kobayashi, Yuta Shimoi, Megumi Masuda, Yoshiko Shibata, Naomi Fujiki, Ritsuko Toyama, Chieko Nozawa, Kozaburo Endo, Akashi Koseki, Katsuhide Ikeda, Daisuke Asahina, Kazuki Ito, Asuka Takaku, Momoyo Ogawa, Shigeru Tamura, Megumi Miura, Mikako Kobayashi
Routine activities / Research activities
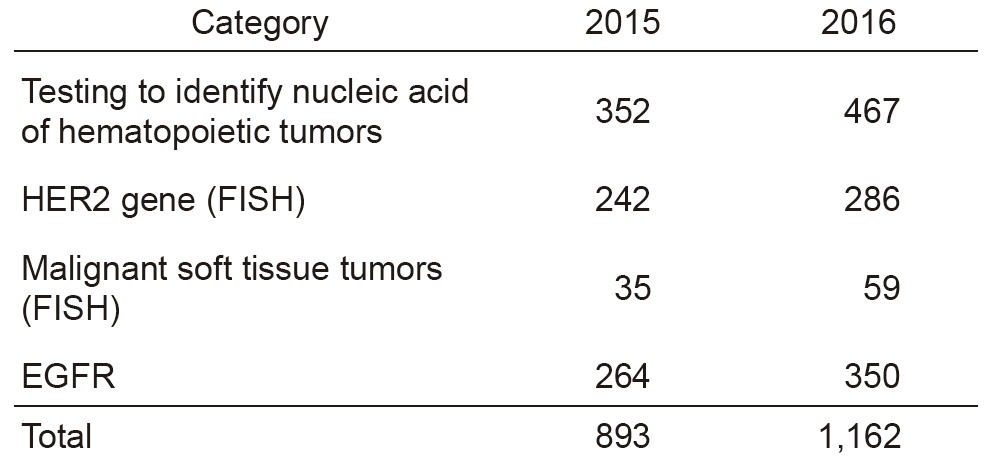
The services of the Clinical Laboratories are organized into five sections: in vitro diagnostics (IVD) (general, hematology, biochemistry, immunology, bacteriology, and gene testing), transfusion and cell therapy testing, blood sampling, physiological examination, and pathology diagnosis. We have achieved re-accreditation to ISO 15189, the international standard for quality management and technical competence in medical laboratories. In the IVD Section, we have undertaken a complete renewal of biochemistry, immunology, and general testing instruments, as well as IVD and specimen dispensing transport systems, in order to provide quick and accurate testing results in examinations. In response to an increase in the number of sampled patients, we have added two booths in the central blood-drawing room which increased to the total of nine booths to reduce waiting time. In the Transfusion and Cell Therapy Testing Section, we determine the timing for sampling autologous-allogeneic peripheral blood stem cells using HPC, that contributes to reducing the burden on patients and donors, and to achieving better safety. We have also upgraded flow cytometry to improve its ability for quick report of the number of cells collected. In the Pathology Examination Section, we have introduced an automatic microtome to handle the increasing number of specimens. In the Physiological Examination Section, the number of cardiac ultrasonography and preoperative leg vein ultrasonography tests for clinical trials have been increasing. In the Gene Testing Section, we have begun comprehensive gene testing using a next-generation sequencer (NGS) in collaboration with Sysmex Corporation at the Sysmex Cancer Innovation Laboratory (SCI-Lab), and the number of cases is steadily increasing. In 2016, the total number of clinical tests performed was 6,123,975, recording a 6% increase from the previous year. In particular, gene tests increased by 30% (See Tables 1 and 2).
Table 1. Number of clinical tests performed
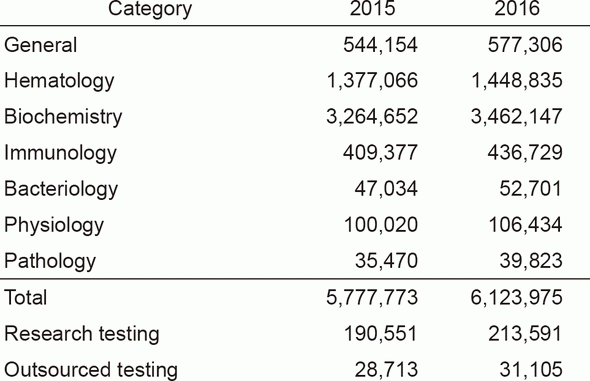
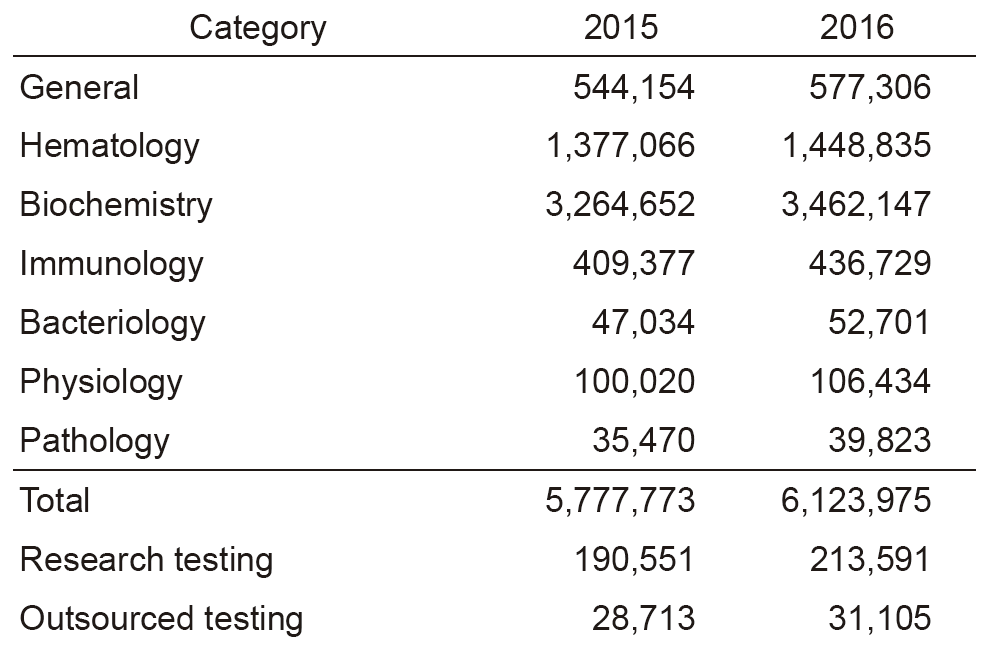
Table 2. Number of gene tests performed

We are also cooperating in the promotion of academy-industry-government joint research, clinical trials, and biobank projects.
Research achievement
We are pursuing initiatives toward enhanced precision and standardization in gene testing.
Education
We conduct our own training regarding blood sampling. We perform extensive ongoing technological training, including patient reception for sampling, handling of specimens, responding to mechanical problems, and dealing with patients. In principle, young technologists are assigned to specialized departments after mastering the basics of laboratory work in the IVD Section, which is fundamental. With regard to ISO 15189, now that four years have passed since acquiring the certification, we held a workshop focusing on ISO to instill an understanding of the workings of PDCA (plan-do-check-act) cycle among all staff members. As it is essential to enhance the level of individual sections throughout the Clinical Laboratories, monthly study meetings were held and ataff members were encouraged to participate in and give presentions at external seminars and academic conferences. We maintain an ongoing internship program with universities, aiming to promote the appeal of medical laboratory work.
Future prospects
We aim to put operations at the SCI-Lab on track, establish a system in which clinical laboratory technologists should play a role, and become involved in leading-edge clinical development focusing on gene testing. In general medical practice, we intend to provide testing results more quickly in responding to ultrasound examination needs, pathology examinations conducted in line with an increasing number of surgeries and endoscopies, and IVD of increased outpatients. In blood sampling operations, we intend to locate all sampling-related activities, including medical practice, clinical trials and research, in the central blood-drawing room, and aim to reduce the burden on patients. In academics, we work to proactively disseminate information through conference presentations and scientific papers.
