HOME > Publication & Reports > Annual Report 2016 > Research Institute
Division of Carcinogenesis and Cancer Prevention(Viral Carcinogenesis and Prevention Group)
Tohru Kiyono, Takashi Yugawa, Tomomi Nakahara, Kenji Yamada, Ghani Farhana Ishrat, Takako Ishiyama, Yuki Yoshimatsu, Katsuyuki Tanaka, Chiho Kohno, Yuki Inagawa, Shin-ichi Ohno, Yukimi Gotoh, Etsuko Kabasawa
Introduction
Approximately 15% of human cancers have a viral etiology. Research in the Division of Carcinogenesis and Cancer Prevention is mainly focused on the molecular mechanisms of carcinogenesis associated with human papillomavirus (HPV) infection. A subset of HPVs including types 16 and 18 are closely associated with human cancers and have thus been called high-risk HPVs (HR-HPVs). The E6 and E7 proteins of HR-HPVs are known to inactivate the major tumor suppressors, p53 and retinoblastoma protein (pRB), respectively. By using an in vitro multistep carcinogenesis model, we study specific association between oncogenic driver genes and pathological features of cancers with and without a viral etiology.
Our team and what we do
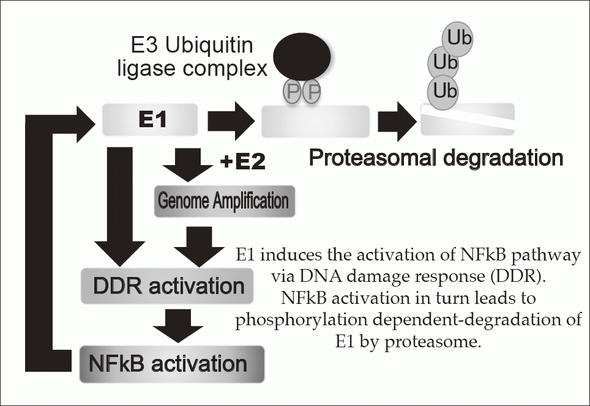
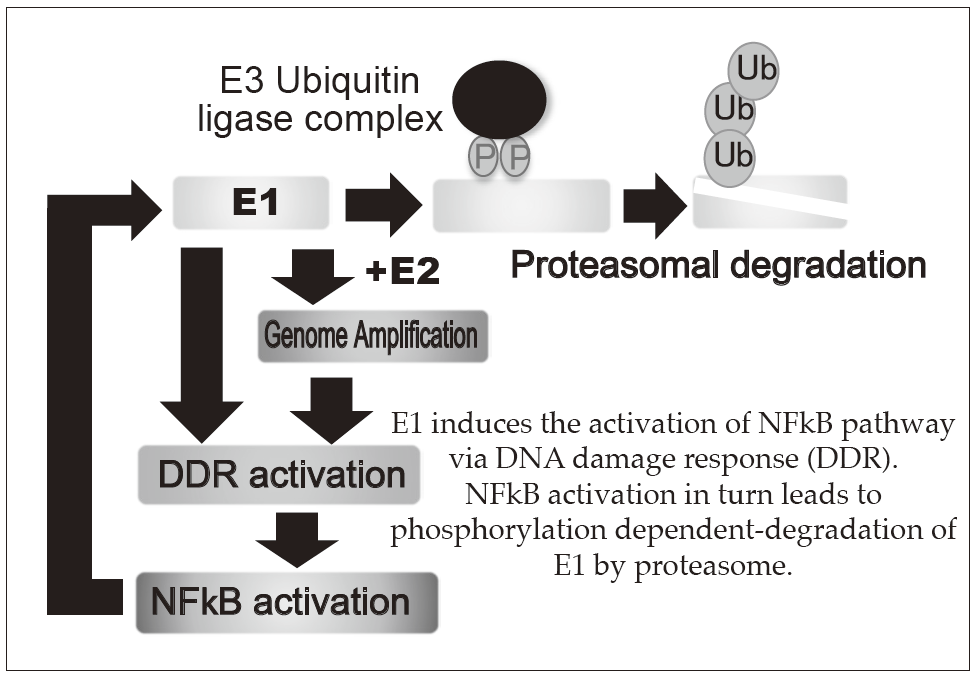
E1 induces the activation of NFkB pathway
via DNA damage response (DDR).
NFkB activation in turn leads to phosphorylation dependent-degradation of E1 by proteasome.
To clarify molecular mechanisms of oncogenesis by viral and cellular oncogenes and inactivation of tumor suppressors, we are establishing ex vivo carcinogenesis models for cervical cancer and other cancers by transducing abnormalities of genes found in cancer into normal cells-of-origin of each cancer.
Research activities
1.HPV-induced carcinogenesis and its prevention
Persistent infection of the HR-HPVs is responsible for 5% of all cancers with 11% of woman cancers worldwide, which include cervical, anal, vaginal, vulvar, penile, and head and neck cancers. The proliferation of HPV depends on the differentiation of mucosal and cutaneous epithelia. In basal cells, viral genomes are maintained as low copy number-episomes and expression of the viral genes is very limited. This latent state in basal cells is thought to facilitate persistent infection which can extend to a few decades by eluding host immune surveillance. The development of anti-HPV drugs and/or therapeutic vaccines to eradicate the viral genomes and/or the infected cells can realize non-invasive treatment to prevent HPV associated cancers. The primary focus of our research is to elucidate the molecular mechanisms of the viral genome replication and to develop anti-HPV drugs. We established a tissue culture model for HPV infection by generating a human cervical keratinocyte (HCK) cell line that maintains espisomal HPV16 genomes. By using this model, we previously demonstrated that the viral helicase E1 is dispensable for maintenance replication of the HPV genome in basal cells. Subsequent studies indicated that high E1 expression induces activation of NFκB pathway and NFκB functions as a negative feedback by facilitating phosphorylation dependent- proteasomal degradation of E1 (Figures 1 and 2). We propose that this negative feedback loop is a regulatory mechanism to limit an E1 level in latent state of HPV infection. Currently, we are further analyzing an E1-independent mechanism of the viral maintenance in basal cells and a possible link between disruption of the E1-NFκB feedback loop and neoplastic progression of precancerous cervical lesions. In addition, we have developed HCK lines maintaining recombinant HPV16 "reporter" genome which express a reporter gene as episomes and now evaluating the validity of this cell line as a platform for anti-HPV drug screening. Recent genome editing technology with nucleases such as Zinc finger nuclease and CRISPR/Cas made it possible to directly target HPV genome. We also began to test the therapeutic potential of targeting E6/E7 regions of HPV16 and 18 by the CRISPR/Cas system.
2.In vitro human carcinogenesis model
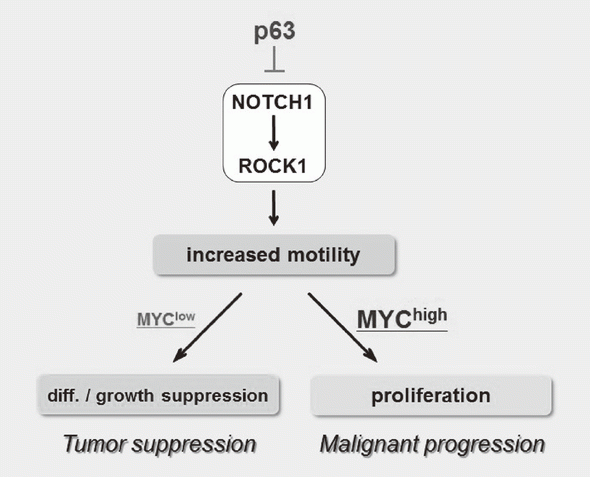
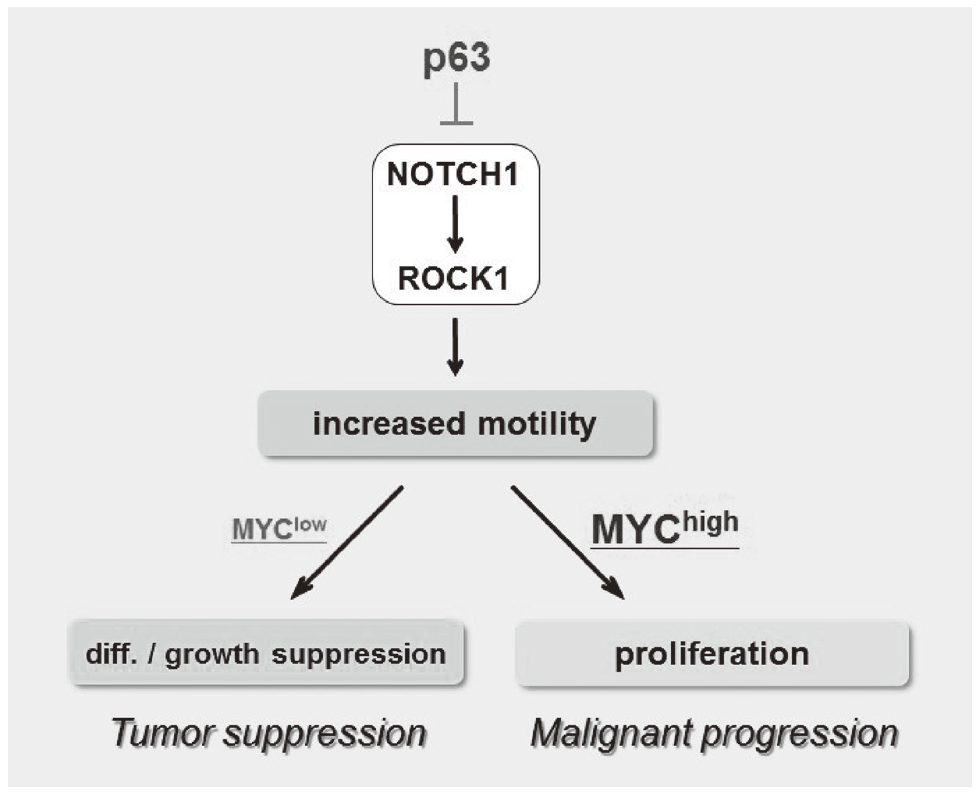
We have been studying the biological significance of p63 expression and the molecular basis underlying acquisition of malignant traits in squamous cell carcinomas (SCCs) of cervical and head and neck cancers. p63 is a master regulator for the development of stratified epithelium and has a role in stem cell maintenance. In line with its physiological role, p63 is frequently overexpressed in more than 50% of human SCCs including examples in the cervix, lung, and head and neck. Paradoxically, loss of p63 is shown to be associated with metastasis appearance and poor prognosis in SCCs, although the underlying molecular mechanism and its functional relevance to carcinogenesis remains unclear as is the case with NOTCH1. We have identified the novel NOTCH-ROCK pathway downstream of p63. Using our in vitro human multistep carcinogenesis model for cervical cancer, we revealed that knock-down of p63 conferred increased invasiveness through the NOTCH-ROCK pathway in MYC-overexpressing cells (Figure 3). We also confirmed the tendency of mutual exclusivity between p63 and MYC expressions in head and neck SCCs by TCGA analysis. We aim to develop novel strategies for the diagnosis and molecularly targeted therapy against poorly-differentiated SCCs based on its cancer biology.
Education
Two PhD students from Thailand and a Japanese PhD student worked as trainees in our lab and had cancer research training.
Future prospects
To clear HPV infection from CIN lesions, the possible strategy is an eradication of HPV genome with a specific inhibitor of HPV replication or elimination of HPV-infected cells with surgery or by induction of cell death with a drug or a therapeutic vaccine. Elucidation of the transition mechanism from initial amplification to maintenance replication will facilitate the development of such drugs. If such drugs are developed, prevention of cervical cancer will be much easier.
The in vitro carcinogenesis model for given cancer can be established in vitro. Such models will be useful for preclinical assessment of new cancer therapies.
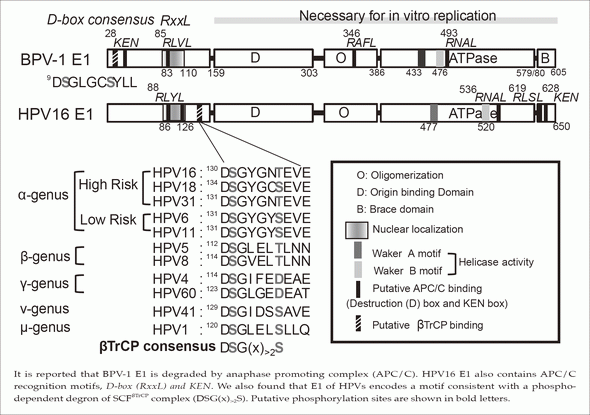
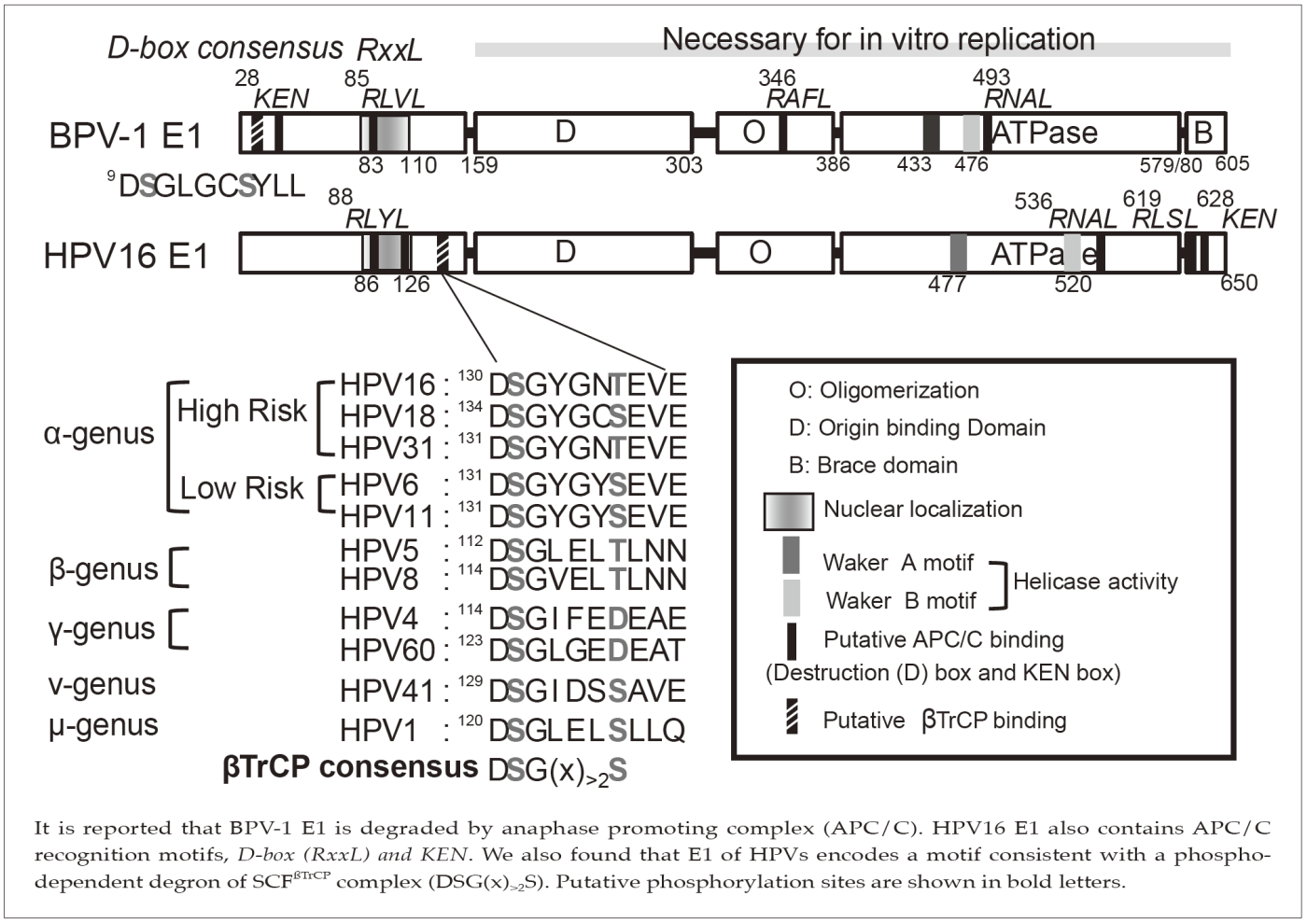
It is reported that BPV-1 E1 is degraded by anaphase promoting complex (APC/C). HPV16 E1 also contains APC/C recognition motifs, D-box(RxxL) and KEN. We also found that E1 of HPVs encodes a motif consistent with a phospho-dependent degron of SCFsTrCP complex (DSG(x)>2S). Putative phosphorylation sites are shown in bold letters.
List of papers published in 2016
Journal
1.Yachida S, Wood LD, Suzuki M, Takai E, Totoki Y, Kato M, Luchini C, Arai Y, Nakamura H, Hama N, Elzawahry A, Hosoda F, Shirota T, Morimoto N, Hori K, Funazaki J, Tanaka H, Morizane C, Okusaka T, Nara S, Shimada K, Hiraoka N, Taniguchi H, Higuchi R, Oshima M, Okano K, Hirono S, Mizuma M, Arihiro K, Yamamoto M, Unno M, Yamaue H, Weiss MJ, Wolfgang CL, Furukawa T, Nakagama H, Vogelstein B, Kiyono T, Hruban RH, Shibata T. Genomic Sequencing Identifies ELF3 as a Driver of Ampullary Carcinoma. Cancer Cell, 29:229-240, 2016
2.Chopjitt P, Pientong C, Sunthamala N, Kongyingyoes B, Haonon O, Boonmars T, Kikawa S, Nakahara T, Kiyono T, Ekalaksananan T. E6D25E, HPV16 Asian variant shows specific proteomic pattern correlating in cells transformation and suppressive innate immune response. Biochem Biophys Res Commun, 478:417-423, 2016
3.Nakahara T, Kiyono T. Interplay between NF-κB/interferon signaling and the genome replication of HPV. Future Virol, 11:141-155, 2016
4.Fukuda T, Iino Y, Eitsuka T, Onuma M, Katayama M, Murata K, Inoue-Murayama M, Hara K, Isogai E, Kiyono T. Cellular conservation of endangered midget buffalo (Lowland Anoa, Bubalus quarlesi) by establishment of primary cultured cell, and its immortalization with expression of cell cycle regulators. Cytotechnology, 68:1937-1947, 2016
5.Fukuda T, Iino Y, Onuma M, Gen B, Inoue-Murayama M, Kiyono T. Expression of human cell cycle regulators in the primary cell line of the African savannah elephant (loxodonta africana) increases proliferation until senescence, but does not induce immortalization. In Vitro Cell Dev Biol Anim, 52:20-26, 2016
6.Inaba H, Goto H, Kasahara K, Kumamoto K, Yonemura S, Inoko A, Yamano S, Wanibuchi H, He D, Goshima N, Kiyono T, Hirotsune S, Inagaki M. Ndel1 suppresses ciliogenesis in proliferating cells by regulating the trichoplein-Aurora A pathway. J Cell Biol, 212:409-423, 2016
7.Iwata Y, Suzuki N, Ohtake H, Kamauchi S, Hashimoto N, Kiyono T, Wakabayashi S. Cancer cachexia causes skeletal muscle damage via transient receptor potential vanilloid 2-independent mechanisms, unlike muscular dystrophy. J Cachexia Sarcopenia Muscle, 7:366-376, 2016
8.Kanda T, Furuse Y, Oshitani H, Kiyono T. Highly Efficient CRISPR/Cas9-Mediated Cloning and Functional Characterization of Gastric Cancer-Derived Epstein-Barr Virus Strains. J Virol, 90:4383-4393, 2016
9.Katayama M, Hirayama T, Horie K, Kiyono T, Donai K, Takeda S, Nishimori K, Fukuda T. Induced Pluripotent Stem Cells With Six Reprogramming Factors From Prairie Vole, Which Is an Animal Model for Social Behaviors. Cell Transplant, 25:783-796, 2016
10.Katayama M, Kiyono T, Horie K, Hirayama T, Eitsuka T, Kuroda K, Donai K, Hidema S, Nishimori K, Fukuda T. Establishment of an immortalized cell line derived from the prairie vole via lentivirus-mediated transduction of mutant cyclin-dependent kinase 4, cyclin D, and telomerase reverse transcriptase. Exp Anim, 65:87-96, 2016
11.Kinebuchi M, Matsuura A, Kiyono T, Nomura Y, Kimura S. Diagnostic copper imaging of Menkes disease by synchrotron radiation-generated X-ray fluorescence analysis. Sci Rep, 6:33247, 2016
12.Kondo T, Ozawa S, Ikoma T, Yang XY, Kanamori K, Suzuki K, Iwabuchi H, Maehata Y, Miyamoto C, Taguchi T, Kiyono T, Kubota E, Hata RI. Expression of the chemokine CXCL14 and cetuximab-dependent tumour suppression in head and neck squamous cell carcinoma. Oncogenesis, 5:e240, 2016
13.Mizushima T, Asai-Sato M, Akimoto K, Nagashima Y, Taguri M, Sasaki K, Nakaya M-a, Asano R, Tokinaga A, Kiyono T, Hirahara F, Ohno S, Miyagi E. Aberrant Expression of the Cell Polarity Regulator aPKClambda/iota is Associated With Disease Progression in Cervical Intraepithelial Neoplasia (CIN): A Possible Marker for Predicting CIN Prognosis. Int J Gynecol Pathol, 35:106-117, 2016
14.Pantic B, Borgia D, Giunco S, Malena A, Kiyono T, Salvatori S, De Rossi A, Giardina E, Sangiuolo F, Pegoraro E, Vergani L, Botta A. Reliable and versatile immortal muscle cell models from healthy and myotonic dystrophy type 1 primary human myoblasts. Exp Cell Res, 342:39-51, 2016
15.Tone H, Yoshioka S, Akiyama H, Nishimura A, Ichimura M, Nakatani M, Kiyono T, Toyoda M, Watanabe M, Umezawa A. Embryoid Body-Explant Outgrowth Cultivation from Induced Pluripotent Stem Cells in an Automated Closed Platform. Biomed Res Int, 2016:7098987, 2016
16.Yoshida M, Taguchi A, Kawana K, Adachi K, Kawata A, Ogishima J, Nakamura H, Fujimoto A, Sato M, Inoue T, Nishida H, Furuya H, Tomio K, Arimoto T, Koga K, Wada-Hiraike O, Oda K, Nagamatsu T, Kiyono T, Osuga Y, Fujii T. Modification of the Tumor Microenvironment in KRAS or c-MYC-Induced Ovarian Cancer-Associated Peritonitis. PLoS One, 11:e0160330, 2016