HOME > Publication & Reports > Annual Report 2016 > Research Institute
Division of Cancer Stem Cell
Kenkichi Masutomi, Yoshiko Maida, Mami Yasukawa, Marco Ghilotti, Tomoko Hayakawa, Akiya Tatsumi
Introduction
Research in the Division of Cancer Stem Cell is focused on deciphering the mechanisms that establish and maintain cancer stem cells, and developing novel therapeutic approaches to treat cancer stem cells. In particular, our division studies the molecular links between a) telomerase and RNA-dependent RNA polymerase (RdRP); b) TERT and cancer stem cells; and c) RdRP and anticancer drugs.
Research activities
1. Telomerase and RNA-dependent RNA polymerase
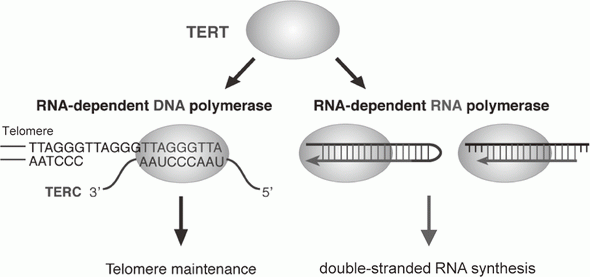
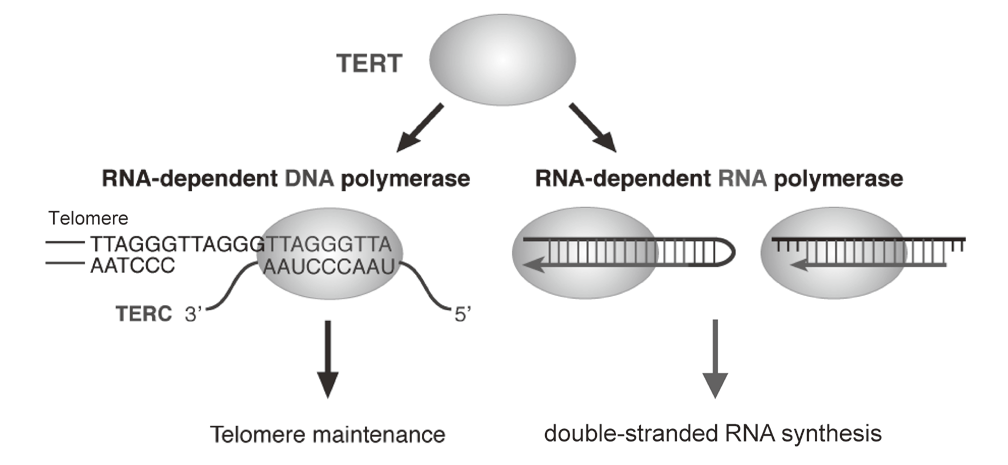
Telomerase is a ribonucleoprotein complex that elongates telomeres. Human TERT (telomerase reverse transcriptase) is known as the catalytic subunit of the enzyme. TERT acts as an RNA-dependent DNA polymerase (RdDP) and synthesizes telomere DNA from an RNA template TERC. Although the major function of TERT is believed to be telomere elongation, emerging evidence indicates that TERT exhibits various functions beyond telomere maintenance. We reported that TERT has an RdRP activity and synthesizes double-stranded RNA in either a primer-dependent or a primer-independent manner1, 2 (Figure 1). The RdRP enzyme complex is distinct from telomerase; TERT assembles with BRG1 and nucleostemin (NS), and the TERT-BRG1-NS complex (TBN complex) exerts RdRP activity3.
RdRPs in yeast and worm regulate centromeric heterochromatin formation, and RdRPs are required for proper chromosome segregation during mitosis in these organisms. The RNA-directed RNA polymerase complex (RDRC) contributes to the regulation, and the complex contains RdRP and RNA helicase. Because TERT has RdRP activity, and BRG1 has helicase activity, we speculated that the TBN complex might have similar functions with the RDRC. We confirmed that the TBN complex suppresses transcription from heterochromatic regions at centromeres and transposons, and suppression of the TBN complex results in the increase of the cells arrested in mitosis and the heterochromatic transcription3. These observations indicate that the TBN complex contributes to mitotic progression through the regulation of heterochromatin maintenance. Our findings suggest that the novel functions of TERT might be a promising target for development of anticancer strategies.
2. TERT and cancer stem cells
Previous studies indicated that TERT has activities beyond telomere maintenance, and it is speculated that the constitutive expression of TERT not only stabilizes telomere length and facilitates cell immortalization but also contributes to tumor susceptibility and alters stem cell cycling in vivo even when telomere lengths are not limited. We found that the TBN complex participates in the regulation of tumor initiating cells (TICs) phenotypes through telomere-independent mechanisms4. We also confirmed that the cells that constitutively express NS exhibited increased beta-catenin signaling and elevated MYC, OCT3/4, KLF4, and TWIST expression. Moreover, cells that constitutively express elevated levels of TERT, BRG1, and NS exhibit increased CD133 and CD44 expression and enhanced tumorigenicity at limiting cell numbers. These observations indicate that the TBN complex is essential for the maintenance of TICs.
3. RdRP and anticancer drugs
We screened a series of anti-cancer compounds for growth suppression of ovarian cancer cell lines and found that eribulin mesylate (eribulin) effectively inhibits the growth of platinum-resistant ovarian cancer cells5. Although, it has been confirmed that eribulin exerts its anticancer effect by blocking the elongation of microtubules, we found that eribulin specifically inhibits the RdRP activity of TERT in vitro, suggesting TERT-RdRP as a novel molecular target of the drug beyond tubulin. This hypothesis was further supported by the results showing that 1) eribulin-sensitive ovarian cancer cell lines express high levels of TERT, and 2) suppression of TERT expression reduced sensitivity to eribulin. The eribulin-sensitive cell lines have enhanced cancer stem cell (CSC)-like traits, the characteristics related to TERT, as well. Our study demonstrated that eribulin might be a promising therapeutic agent for platinum-resistant ovarian cancer. We further confirmed that TERT protein levels and TERT-associated RdRP activity are positively correlated in various human cancer cell lines2, indicating that eribulin may work effectively for many types of tumors with high-levels of TERT.
References
1.Maida Y, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature, 461:230-235, 2009.
2.Maida Y, et al. De novo RNA synthesis by RNA-dependent RNA polymerase activity of TERT. Mol Cell Biol, 36:1248-1259, 2016
3.Maida Y, et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol Cell Biol, 34:1576-1593, 2014.
4.Okamoto N, et al. Maintenance of tumor initiating cells of defined genetic composition by nucleostemin. Proc Natl Acad Sci U S A, 108:20388-20393, 2011.
5.Yamaguchi S, et al. Eribulin mesylate targets human telomerase reverse transcriptase in ovarian cancer cells. PLOS ONE, 9:e112438, 2014.
List of papers published in 2016
Journal
1.Maida Y, Yasukawa M, Masutomi K. De Novo RNA Synthesis by RNA-Dependent RNA Polymerase Activity of Telomerase Reverse Transcriptase. Mol Cell Biol, 36:1248-1259, 2016
2.Neri S, Hashimoto H, Kii H, Watanabe H, Masutomi K, Kuwata T, Date H, Tsuboi M, Goto K, Ochiai A, Ishii G. Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. J Cancer Res Clin Oncol, 142:437-446, 2016