HOME > Publication & Reports > Annual Report 2016 > Research Institute
Division of Chemotherapy and Clinical Research
Tesshi Yamada, Mitsuko Masutani, Masaya Ono, Kazufumi Honda, Mari Masuda, Hiroaki Fujimori-Sakuma, Nami Miura, Naoko Goto, Kaoru Onidani, Shoji Imamichi, Haruyo Tozaki, Keiko Takeuchi, Teppei Sugano, Toru Hirozane, Yuka Sasaki, Akira Sato, Makoto Ihara, Takae Onodera, Kumiko Kinoshita, Hiroki Maruta, Junhui Wang
Our team and what we do
Development of new cancer therapeutics and diagnostics.
1.Discovery of a novel compound that abrogates colorectal cancer stemness
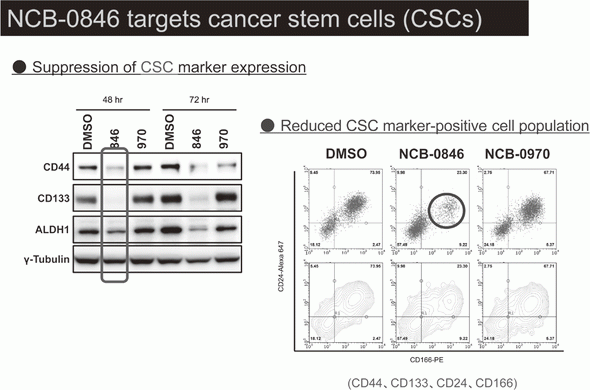
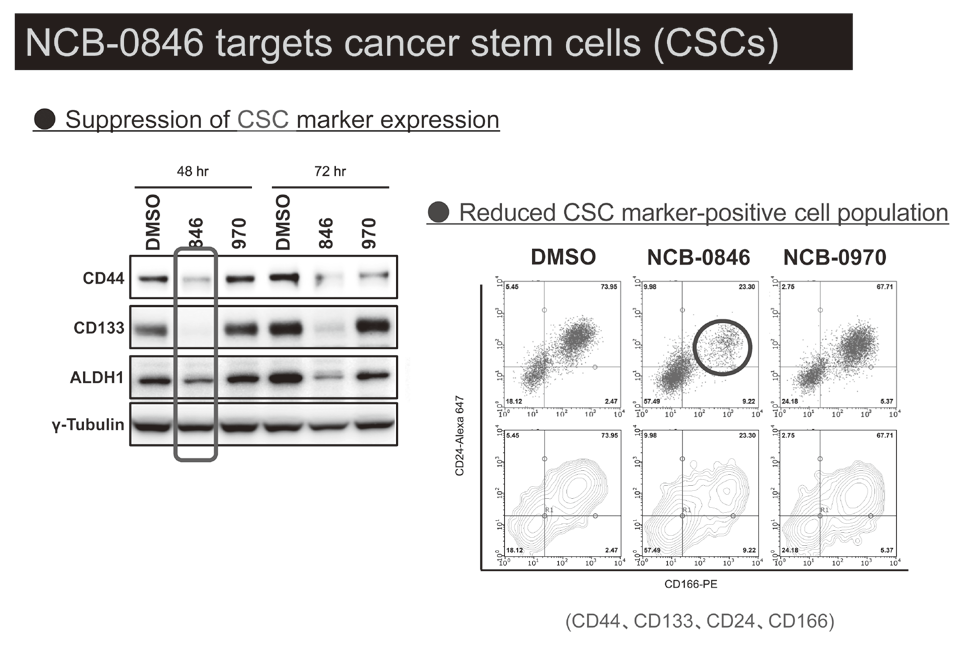
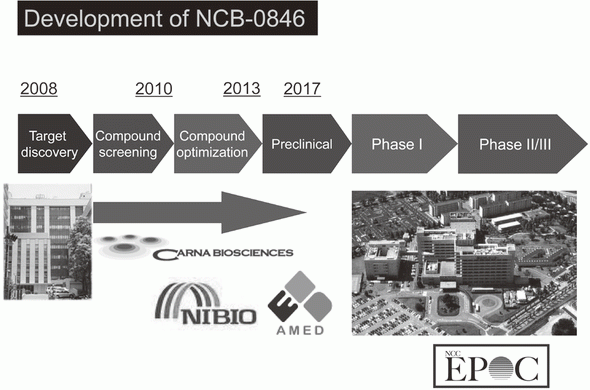
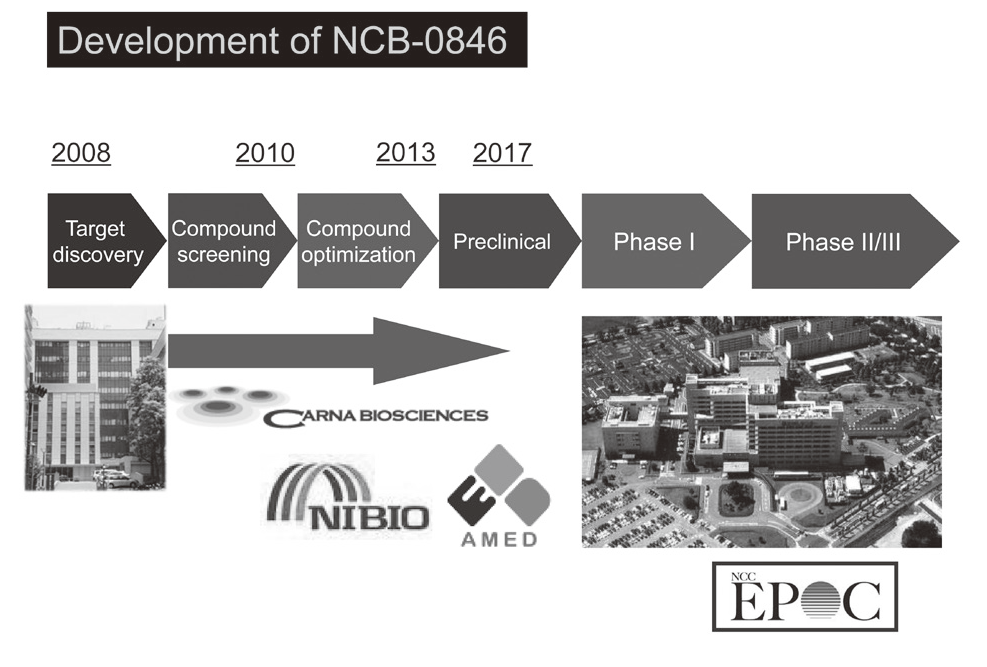
Canonical Wnt/s-catenin signaling is essential for maintaining intestinal stem cells, and genetic alterations in this signaling have been implicated in colorectal carcinogenesis. We and others have previously identified Traf2- and Nck-interacting kinase (TNIK) as a regulatory component of the T-cell factor-4 (TCF4) and s-catenin transcriptional complex. TNIK is essential for Wnt signaling and colorectal cancer growth, and it has been suggested that pharmacological intervention to alter its function might abrogate cancer stemness and thus be a treatment for colorectal cancer. We screened a kinase-focused compound library in collaboration with Carna BioSciences Inc. (Kobe, Japan) and identified a series of quinazoline analogues with high TNIK enzyme-inhibitory activity. Subsequent lead optimization resulted in the identification of NCB-0846. NCB-0846 inhibited the TCF/LEF transcriptional activity of colorectal cancer cells, and Wnt-driven intestinal tumorigenesis in Apcmin/+ mice. NCB-0846 reduced the proportion of cells showing high expression of cancer stem cell surface markers and aldehyde dehydrogenase activity (Figure 1). NCB-0846 was orally administrable and suppressed the growth of different patient-derived colorectal cancer xenografts. The compound is now undergoing preclinical safety evaluation aimed at a phase 1 clinical trial (Figure 2).
2.Efficacy of adjuvant chemotherapy for non-small cell lung cancer
Although several clinical trials have demonstrated the benefits of platinum-combined adjuvant chemotherapy for stage II and stage IIIA non-small cell lung cancer (NSCLC), predictive biomarkers for the efficacy of such therapy have yet to be identified. Moreover, the clinical benefit of such therapy for overall survival has not been observed for stage-IB cancer.
By analyzing patient data obtained from a public database created by JBR.10, we have found evidence supporting an interaction between overexpression of ACTN4 in stage-IB/II patients with NSCLC, and the efficacy of adjuvant chemotherapy in NSCLC.
3.Plasma biomarker for detection of early stage pancreatic cancer
Because of the low incidence of pancreatic cancer, it is generally considered that the screening of the general population for pancreatic cancer using invasive and expensive modalities is difficult to take into consideration for the cost effectiveness.
We recently reported that circulating apolipoprotein A2 (apoA2) isoforms apoA2-ATQ/AT (C-terminal truncations of the apoA2 homo-dimer) decline significantly in pancreatic cancer and thus might serve as plasma biomarkers for the early detection of this disease. We developed novel enzyme-linked immunosorbent assays (ELISAs) for measurement of apoA2-ATQ/AT, and evaluated their clinical applicability for early detection of pancreatic cancer. Plasma and serum concentrations of apoA2-ATQ/AT were measured in three independent cohorts, which comprised healthy control subjects, and patients with pancreatic cancer and gastroenterologic diseases (n = 1156). These cohorts included 151 cases of stage I/II pancreatic cancer. ApoA2-ATQ/AT not only distinguished the early stages of pancreatic cancer from healthy controls, but also identified patients at high risk for pancreatic malignancy. The area under curve (AUC) values of apoA2-ATQ/AT to detect early stage pancreatic cancer were higher than those of CA19-9 in all independent cohorts. ApoA2-ATQ/AT is a potential biomarker for screening patients in the early stage of pancreatic cancer and identifying patients at risk for pancreatic malignancy. Combination strategies with biomarkers of apoA2-isoforms and imaging are attractive for pancreatic cancer screening.
4.Boron-captured neutron therapy
Accelerator-based BNCT (boron neutron- capture therapy) system has been developed as a collaborative project of the NCC and industries. The biological evaluation of this BNCT system using cell lines has been carried out using 10B- para-boronophenylalamine (BPA). For optimization of BNCT, cell death mechanism, immune and inflammatory responses have been characterized after boron neutron-capture reaction (BNCR) using cancer cell lines.
5.Evaluation of radiosensitization targets
PARP inhibitor olaparib was shown to be effective in sensitization of proton beam irradiation damage by delaying DNA repair responses. The validation of candidate target genes from comprehensive genome-wide screening for radiosensitization has been conducted and mechanistic studies were carried out.
6.The studies on biomarkers for anti-cancer drugs targeting genome
Inhibitors of poly(ADP-ribose) glycohydrolase (PARG) cause poly(ADP-ribose) accumulation and inducecell death. Evaluation of anti-tumor effect of PARG inhibitors has been conducted using mouse xenograft models. For refractory stomach cancers, DNA repair factor ERCC1 is known as a candidate biomarker and the underlying mechanism has been investigated.
List of papers published in 2016
Journal
1.Masuda M, Uno Y, Ohbayashi N, Ohata H, Mimata A, Kukimoto-Niino M, Moriyama H, Kashimoto S, Inoue T, Goto N, Okamoto K, Shirouzu M, Sawa M, Yamada T. TNIK inhibition abrogates colorectal cancer stemness. Nat Commun, 7:12586, 2016
2.Yoneyama T, Ohtsuki S, Honda K, Kobayashi M, Iwasaki M, Uchida Y, Okusaka T, Nakamori S, Shimahara M, Ueno T, Tsuchida A, Sata N, Ioka T, Yasunami Y, Kosuge T, Kaneda T, Kato T, Yagihara K, Fujita S, Huang W, Yamada T, Tachikawa M, Terasaki T. Identification of IGFBP2 and IGFBP3 As Compensatory Biomarkers for CA19-9 in Early-Stage Pancreatic Cancer Using a Combination of Antibody-Based and LC-MS/MS-Based Proteomics. PLoS One, 11:e0161009, 2016
3.Honda K, Srivastava S. Potential usefulness of apolipoprotein A2 isoforms for screening and risk stratification of pancreatic cancer. Biomark Med, 10:1197-1207, 2016
4.Wang J, Ding Q, Fujimori H, Motegi A, Miki Y, Masutani M. Loss of CtIP disturbs homologous recombination repair and sensitizes breast cancer cells to PARP inhibitors. Oncotarget, 7:7701-7714, 2016
5.Yasukawa M, Fujihara H, Fujimori H, Kawaguchi K, Yamada H, Nakayama R, Yamamoto N, Kishi Y, Hamada Y, Masutani M. Synergetic effects of PARP inhibitor AZD2281 and cisplatin in oral squamous cell carcinoma in vitro and in vivo. Int J Mol Sci, 17:272, 2016
6.Sato A, Omi T, Yamamoto A, Satake A, Hiramoto A, Masutani M, Tanuma S-i, Wataya Y, Kim H-S. MicroRNA-351 Regulates Two-Types of Cell Death, Necrosis and Apoptosis, Induced by 5-fluoro-2'-deoxyuridine. PLoS One, 11:e0153130, 2016
7.Hirai T, Saito S, Fujimori H, Matsushita K, Nishio T, Okayasu R, Masutani M. Radiosensitization by PARP inhibition to proton beam irradiation in cancer cells. Biochem Biophys Res Commun, 478:234-240, 2016
8.Sasaki Y, Hozumi M, Fujimori H, Murakami Y, Koizumi F, Inoue K, Masutani M. PARG Inhibitors and Functional PARG Inhibition Models. Curr Protein Pept Sci, 17:641-653, 2016
9.Osada T, Nozaki T, Masutani M. Parp1 Deficiency Confers Defects in Chromatin Surveillance and Remodeling During Reprogramming by Nuclear Transfer. Curr Protein Pept Sci, 17:693-704, 2016
10.Sawa M, Masuda M, Yamada T. Targeting the Wnt signaling pathway in colorectal cancer. Expert Opin Ther Targets, 20:419-429, 2016
11.Shiba S, Morizane C, Hiraoka N, Sasaki M, Koga F, Sakamoto Y, Kondo S, Ueno H, Ikeda M, Yamada T, Shimada K, Kosuge T, Okusaka T. Pancreatic neuroendocrine tumors: A single-center 20-year experience with 100 patients. Pancreatology, 16:99-105, 2016
12.Takahashi N, Iwasa S, Fukahori M, Sudo K, Sasaki Y, Shoji H, Honma Y, Okita NT, Takashima A, Hamaguchi T, Boku N, Shimada Y, Honda K, Yamada T, Yamada Y. A phase I study of the combination of panitumumab and bevacizumab in KRAS wild-type colorectal cancer patients previously treated with fluoropyrimidine, oxaliplatin, irinotecan and bevacizumab. Cancer Chemother Pharmacol, 78:567-575, 2016
13.Miura N, Kamita M, Kakuya T, Fujiwara Y, Tsuta K, Shiraishi H, Takeshita F, Ochiya T, Shoji H, Huang W, Ohe Y, Yamada T, Honda K. Efficacy of adjuvant chemotherapy for non-small cell lung cancer assessed by metastatic potential associated with ACTN4. Oncotarget, 7:33165-33178, 2016