HOME > Publication & Reports > Annual Report 2016 > Research Institute
Division of Cancer Immunology
Hiroyoshi Nishikawa, Yuka Maeda, Kentaro Shinohara, Natsuko Takatsuka, Shizuko Ohno, Tokiyoshi Tanegashima, Joji Nagasaki, Nanae Shimoyama
Introduction
Cancer immunotherapy is becoming one of the important strategies for treating patients with cancer and has opened a new era of cancer therapy based on the successes of several clinical trials and many research findings (Fig. 1). Upon the clinical introduction of cancer immunotherapy in which treatment efficacy is dependent on the immune system, resulting in the partial window of clinical responses, comprehensive cancer research including genome and immunology are urgently required to identify biomarkers that can predict responders for cancer immunotherapies.
Research activities
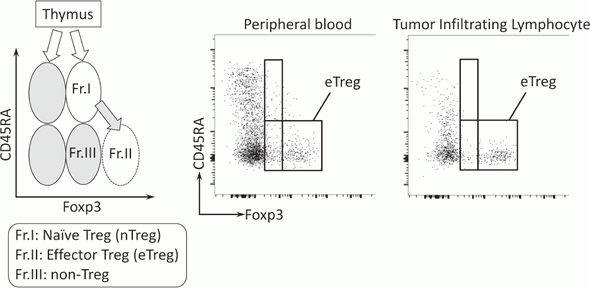
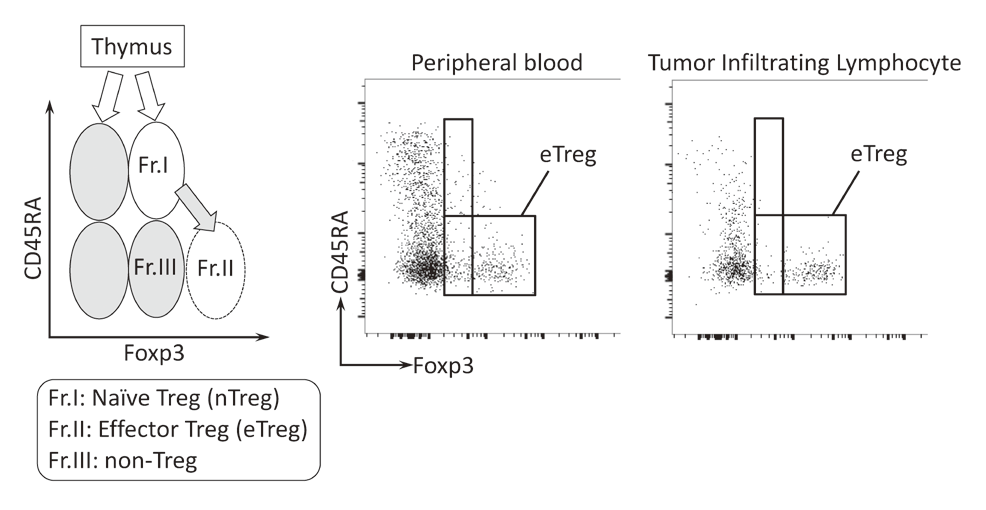
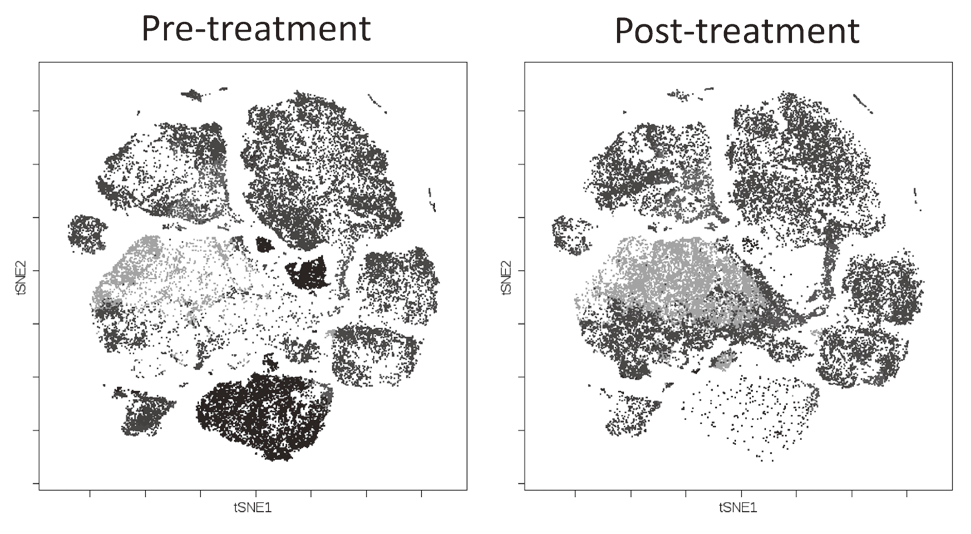
To investigate the immune responses essential for tumor regression, we collected peripheral blood and tumor tissues from surgically resected samples and biopsy samples from various types of cancers including malignant melanoma and lung cancers, pre-/post-treatment of immune checkpoint blockade therapy such as anti-PD-1 mAb and anti-CTLA-4 mAb. In humans, FoxP3 expression is induced in naive CD4+ T cells upon T-cell receptor stimulation, and the presence of this non-regulatory FoxP3-expressing CD4+ T cell population in the periphery has compromised the identification of Tregs. FoxP3+CD4+ T cells can be dissected into three subpopulations: FoxP3loCD45RA+ cells (naive Tregs), FoxP3hiCD45RA- cells (effector Tregs, eTregs), which are terminally differentiated and highly suppressive; and FoxP3loCD45RA-CD25lo non-Tregs, which do not possess suppressive activity but can secrete pro-inflammatory cytokine (Fig. 2).
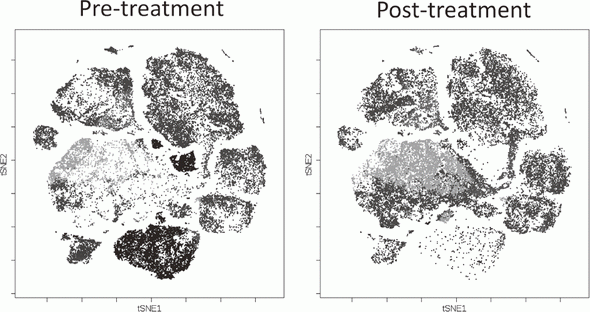
We, particularly, focused on Tregs which have a strong suppressive function in tumor tissues, namely effector Treg (eTreg). We are evaluating these Treg subsets by examining immune checkpoint molecules and other markers including phosphorylation signals and function by conventional flow cytometry and mass-cytometry (CyTOF) that can analyze more than 30 molecules at once (Fig. 3).
Education
Seven graduate school students (Dermatology, Hematology, Pulmonology, and Urology) are trained in our division and two post-doctoral fellows will join in the autumn of 2017 from the area outside of immunology. We believe that this diversity is helpful for understanding a comprehensive cancer microenvironment which is critical for developing effective cancer immunotherapy.
Future prospects
We integrate conventional immunological technique and genomic data in many cancer types to understand the immune responses essential for tumor regression, resulting in clarifying biomarkers to predict clinical responses of cancer immunotherapies and proposing novel combination therapies. Translational research and reverse-translational research are then important to make medical-innovation to move ahead. The Division of Cancer Immunology will realize the harmony of basic science and medical-innovation.
List of papers published in 2016
Journal
1.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med, 22:679-684, 2016
2.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol, 28:401-409, 2016
3.Ureshino H, Shindo T, Nishikawa H, Watanabe N, Watanabe E, Satoh N, Kitaura K, Kitamura H, Doi K, Nagase K, Kimura H, Samukawa M, Kusunoki S, Miyahara M, Shin-I T, Suzuki R, Sakaguchi S, Kimura S. Effector Regulatory T Cells Reflect the Equilibrium between Antitumor Immunity and Autoimmunity in Adult T-cell Leukemia. Cancer Immunol Res, 4:644-649, 2016
4.Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, Mimura I, Morita H, Sugiyama D, Nishikawa H, Hattori M, Hino Y, Ikegawa S, Yamamoto K, Toya T, Doki N, Koizumi K, Honda K, Ohashi K. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood, 128:2083-2088, 2016
5.Haseda F, Imagawa A, Nishikawa H, Mitsui S, Tsutsumi C, Fujisawa R, Sano H, Murase-Mishiba Y, Terasaki J, Sakaguchi S, Hanafusa T. Antibody to CMRF35-Like Molecule 2, CD300e A Novel Biomarker Detected in Patients with Fulminant Type 1 Diabetes. PLoS One, 11:e0160576, 2016
6.Okubo K, Wada H, Tanaka A, Eguchi H, Hamaguchi M, Tomokuni A, Tomimaru Y, Asaoka T, Hama N, Kawamoto K, Kobayashi S, Marubashi S, Nagano H, Sakaguchi N, Nishikawa H, Doki Y, Mori M, Sakaguchi S. Identification of Novel and Noninvasive Biomarkers of Acute Cellular Rejection After Liver Transplantation by Protein Microarray. Transplant Direct, 2:e118, 2016
7.Hayakawa Y, Kawada M, Nishikawa H, Ochiya T, Saya H, Seimiya H, Yao R, Hayashi M, Kai C, Matsuda A, Naoe T, Ohtsu A, Okazaki T, Saji H, Sata M, Sugimura H, Sugiyama Y, Toi M, Irimura T. Report on the use of non-clinical studies in the regulatory evaluation of oncology drugs. Cancer Sci, 107:189-202, 2016