HOME > Publication & Reports > Annual Report 2016 > Research Institute
Department of Biobank and Tissue Resources
Yae Kanai, Masumi Tanaka, Teiko Yamane, Nobuko Nangi
Activities
The Department of Biobank and Tissue Resources operates the National Cancer Center Biobank under the supervision of the National Cancer Center Biobank Administration Committee (Figure 1).
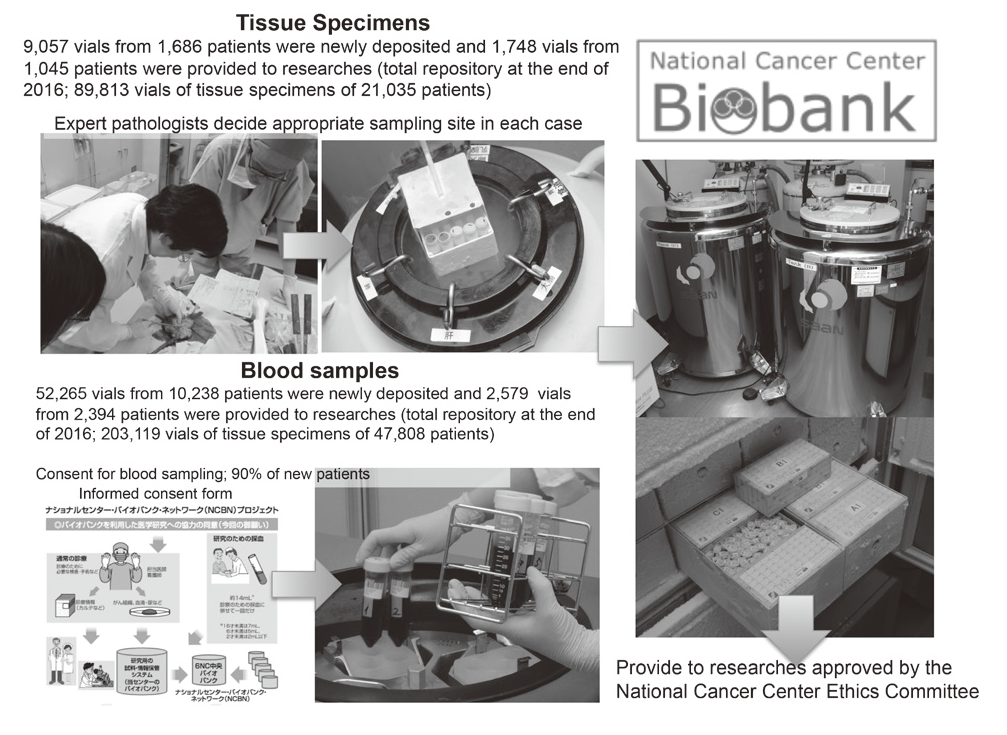
In 2016, 9,057 vials of tissue specimens obtained from surgically resected materials of 1,686 patients were newly deposited at the National Cancer Center Biobank and 1,748 vials of tissue specimens obtained from surgically resected materials of 1,045 patients were provided for researches approved by the National Cancer Center Ethics Committee. The ratio of the number of patients from whom samples were provided for researches to those of whom samples were newly deposited at the Biobank was about 61%. At the end of 2016, we reposited 89,813 vials of tissue specimens of 21,035 patients.
In 2016, 52,265 vials of plasma samples drawn from 10,238 patients were newly deposited at the National Cancer Center Biobank and 2,579 vials of plasma samples drawn from 2,394 patients were provided for researches approved by the National Cancer Center Ethics Committee. At the end of 2016, we reposited 203,119 vials of blood samples of 47,808 patients who consented to give blood samples for research purposes.
Researchers who received samples from the Biobank have published 420 scientific papers (total impact factor; 2,148.254, total citation index; 8,804). A total of 67% of the published papers was based on collaborative researches between researchers of the National Cancer Center and outside researchers; in particular, 18 % of those were based on collaborative researches with industries.
Many founders and/or contact persons of bioresource repositories of other universities and hospitals visited the National Cancer Center Biobank to learn about the management knowhow of the biobank. We have been consulted by contact persons of bioresource repositories of other universities about storage system for specimens. We made efforts to get outside researchers to see appropriate collaborators in the National Cancer Center for collaborative researches using our biobank specimens.
We have built up the catalog database, HosCanR Biobank Edition, by extracting appropriate information from the Interview Sheet Database in a common form among six National Centers in Japan and HosCanR, an application specialized for the National Program of Cancer Registries. Researchers and biobank users can find out samples suitable for their own research plans using the search commands of this catalog database. In 2016, we established the close connection between HosCanR Biobank Edition and the central database of the National Center Biobank Network (NCBN) using an application programming interface. Staff of the National Cancer Center Biobank participate in the Central Steering Committee, Sample Handling System Review Working Group, and Medical Information System Review Working Group of the NCBN.
Future prospects
The continuous collection of samples for various research needs and management of the biobank, including a high-quality clinicopathological information database, are considered to be a national mission. The National Cancer Center Biobank should be continued and become a more robust and permanent research base, it should also continuously support the NCBN and connect the intentions of voluntary donors to next generation personalized medicine.