Annual Report 2017
Division of Developmental Therapeutics
Yasuhiro Matsumura, Masahiro Yasunaga, Yoshikatsu Koga, Hiroki Takashima, Hikaru Iwafuji, Ryou Tsumura, Hirobumi Fuchigami, Takahiro Anzai, Shigehiro Koganemaru, Takuya Shiraishi, Kaori Hayashi, Yohei Tsuruta, Takaki Noguchi, Yoko Omichi, Makoto Wakatsuki, Madoka Nakayama, Mamiko Shimada, Mayumi Yamauchi, Shinji Saijo, Shingo Hanaoka, Masahisa Kudo,
Introduction
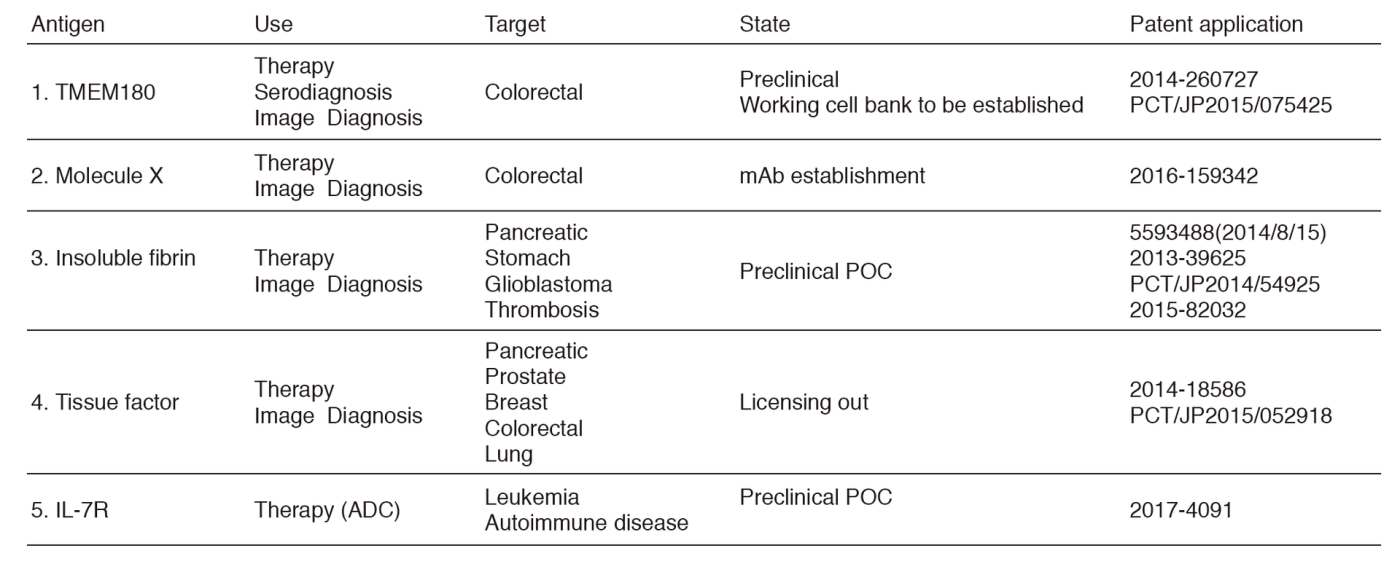
The Division of Developmental Therapeutics has been involved in basic research on drug delivery systems (DDS) and antibody therapeutics including monoclonal antibody (mAb) development, antibody drug conjugate (ADC), and the mAb conjugated micelle system. We have applied patents of all mAbs established in our division (Table 1). We also investigate a mechanism of cancer induced blood coagulation and are developing a new cancer diagnosis based on the cancer specific mAb. In addition to the research works, we are operating the Japan Clinical Oncology Group (JCOG) Tumor Repository.
Our team and what we do
- Examination of clinical trials as an Institutional Review Board (IRB) member
- Operation of the JCOG Tumor Repository
- Management of personal information protection in the National Cancer Center Hospital East (NCCHE)
Research activities
1. Infrastructure for the mAb development
We have established an infrastructure for antibody development including antigen production, animal immunization, hybridoma production, antibody expansion and purification, SPR characterization, and the enzyme-linked immunosorbent assay (ELISA) development. Simultaneously, we have found various cell surface molecules specific to colorectal cancer and succeeded in developing an mAb against one of those molecules.
2. Cancer Stromal Targeting Therapy
In spite of recent success of ADC therapy in patients with hypervascular and special tumors recognized by a particular mAb, there are several issues to be solved for ADC counted as universal therapy for any types of cancer. Especially most human solid tumors possess abundant stroma that hinders the distribution of ADC. To overcome these drawbacks, we developed a unique strategy that the cancer-stromal targeting (CAST) therapy by cytotoxic immunoconjugate bound to the collagen 4, tissue factor (TF), or fibrin network in the tumor stroma from which the payload released gradually and distributed throughout the tumor, resulting in the arrest of tumor growth due to induced damage to tumor cells and tumor vessels.
We successfully developed an mAb (102-10 clone) that reacted only with human fibrin, not with human fibrinogen and cross-reacted with mouse fibrin but not with mouse fibrinogen. The specificity of our 102-10 differs from exiting anti-fibrin mAbs. Namely, our 102-10 reacts only with fibrin clot, but not with fibrinogen, soluble fibrin, or D-dimer. The anti-fibrin antibody therefore did not make immune complex in the bloodstream and circulated in the blood for a long time. We then prepared the ADC that is MMAE conjugated anti-fibrin mAb. The ADC may selectively extravasate from leaky tumor vessels, bind to the fibrin network in the stroma and create a scaffold from which effective sustained release of the free MMAE occurs. This free MMAE may easily reach cancer cells by diffusing through the stromal barrier. Another benefit is that MMAE released from the ADC may also attack the vascular endothelial cells.
3. DDS in Cancer Chemotherapy
Tumor-targeted delivery of therapeutic agents is a longstanding pharmacological goal to improve the treatment selectivity and the therapeutic index. Most scientists have sought to use "active" receptor-mediated tumor-targeting systems. However, the "passive" targeting afforded by the "Enhanced Permeability and Retention (EPR) effect" provides a versatile and non-saturable approach for tumor-selective delivery. Polymeric micelles are ideally suited to exploit the EPR effect, and have been used for the delivery of a range of anticancer drugs in preclinical and clinical studies. Anti-TF mAb conjugated micelle incorporating epirubicin exerted a potent antitumor effect in a human pancreatic cancer model, especially TF-high-expressing tumors.
4. Noninvasive Diagnostic Test for Colorectal Cancer
Regarding colorectal cancer (CRC), we investigated the applicability of the fecal miRNA test (FmiRT) to fecal samples used for a previous fecal occult blood test (FOBT) stored under various conditions.
Education
1) Doctoral students
- Graduate School of Frontier Sciences, The University of Tokyo: two students
- Juntendo University Graduate School of Medicine: one student
- Keio University School of Medicine: one student
- Nihon University School of Medicine: one student
2) Master course student
- Graduate School of Frontier Sciences, The University of Tokyo: four students
Future prospects
The project of our team will be a hybrid of biology and physiology as this topic relates to tumors or tumor stromal biology as well as organic chemistry, and has the potential to establish a new field of drug design and to produce many potentially useful treatment modalities, especially for stroma-rich and refractory cancers such as pancreatic cancer, stomach cancer, CRC, and glioblastoma.
List of papers published in January 2017 - March 2018
Journal
1. Yasunaga M, Manabe S, Matsumura Y. Immunoregulation by IL-7R-targeting antibody-drug conjugates: overcoming steroid-resistance in cancer and autoimmune disease. Sci Rep, 7:10735, 2017
2. Yamazaki N, Koga Y, Taniguchi H, Kojima M, Kanemitsu Y, Saito N, Matsumura Y. High expression of miR-181c as a predictive marker of recurrence in stage II colorectal cancer. Oncotarget, 8:6970-6983, 2017
3. Yasunaga M, Manabe S, Tsuji A, Furuta M, Ogata K, Koga Y, Saga T, Matsumura Y. Development of Antibody-Drug Conjugates Using DDS and Molecular Imaging. Bioengineering (Basel), 4:E78, 2017
4. Koga Y. miRNAs are stable in several severe storage conditions and useful for molecular diagnostics. J Tumor Med Prev, 1:555568, 2017
5. Yamazaki N, Koga Y, Matsumura Y. MicroRNA analysis of colorectal cancer using fecal and tissue samples. RNA Dis, 4:e1592, 2017
6. Takashima H, Tsuji AB, Saga T, Yasunaga M, Koga Y, Kuroda JI, Yano S, Kuratsu JI, Matsumura Y. Molecular imaging using an anti-human tissue factor monoclonal antibody in an orthotopic glioma xenograft model. Sci Rep, 7:12341, 2017
7. Fukuoka S, Kojima T, Koga Y, Yamauchi M, Komatsu M, Komatsuzaki R, Sasaki H, Yasunaga M, Matsumura Y, Doi T, Ohtsu A. Preclinical efficacy of Sym004, novel anti-EGFR antibody mixture, in esophageal squamous cell carcinoma cell lines. Oncotarget, 8:11020-11029, 2017