Annual Report 2017
Department of Head and Neck Medical Oncology
Makoto Tahara, Susumu Okano, Tomohiro Enokida, Yuri Ueda, Takao Fujisawa
Introduction
The Department of Head and Neck Medical Oncology is engaged in the clinical management of patients with head and neck cancer (HNC), and research into anticancer drugs for the treatment of HNC.
Our missions are to: 1) provide the best evidence-based treatment; 2) promote the importance of supportive care in the treatment of patients with HNC; 3) facilitate the timely approval of new drugs by active participation in global clinical trials to eliminate the drug lag; 4) develop cutting-edge treatments; and 5) train experts in head and neck medical oncology.
Our team and what we do
Our department consists of three physicians, one senior resident, and one resident. We manage the treatment of HNC patients who receive anticancer drugs. An estimated 60% of HNC patients require a multidisciplinary approach, including surgery, radiotherapy, and chemotherapy. Furthermore, HNC patients are at risk of injury and impairment of vital organs both from the cancer itself and from the series of treatments provided to cure it. In treating patients, we therefore carefully assess both the curability of the condition and possible subsequent complications, such as swallowing dysfunction and cosmetic changes. Given the increasing complexity of the management of HNC, recommended treatment for patients who are referred to our institution is decided at weekly tumor board meetings attended by a multidisciplinary team.
A total of 461 patients were referred to our department from January 2017 to March 2018 (Tables 1, 2). The outpatient service of our department is available from Monday to Friday. We carefully follow patients during and after treatment and provide palliative chemotherapy as an outpatient service.
Table 1. Number of patients according to sites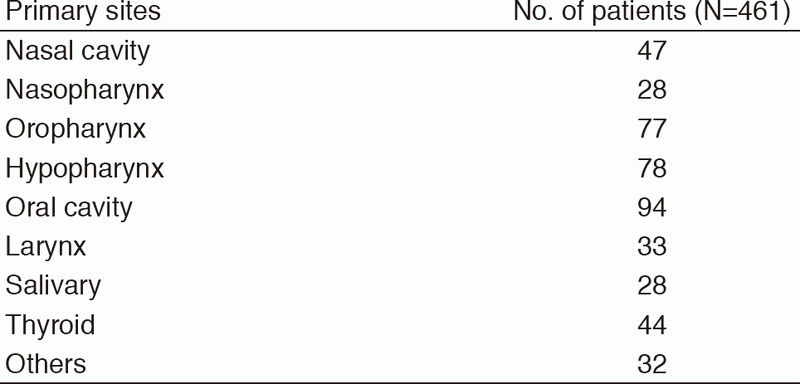
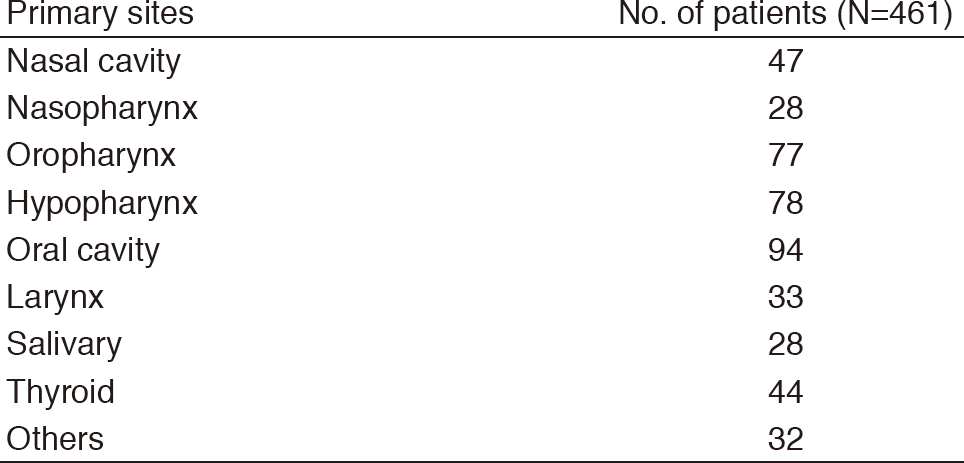
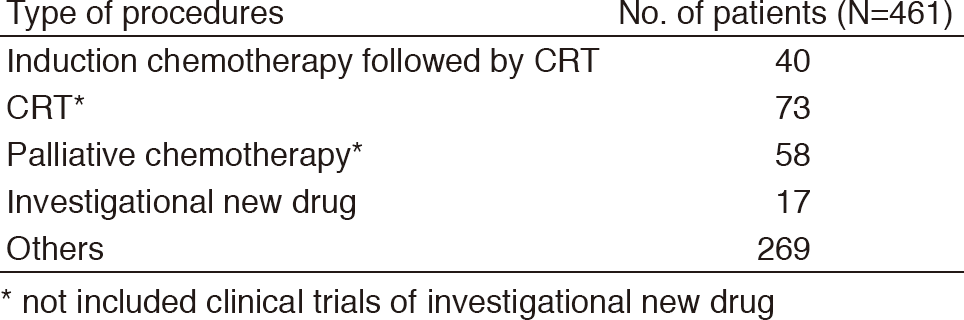
Table 2. Number of patients according to procedures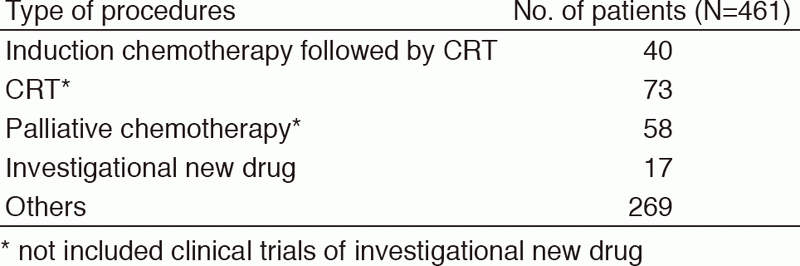
Research activities
Our research activity has focused on three areas, the development of new treatments in clinical trials for HNC, biomarker analysis in HNC, and retrospective analysis of management of treatment for HNC.
1. Development of new treatments
Paclitaxel, carboplatin and cetuximab (PCE) demonstrated promising clinical activity including overall response rate of 40%, median progression-free survival (PFS) of 5.2 months and median overall survival (OS) of 14.7 months in a phase II trial as a first line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) (Tahara M et al., Annals of Oncology, 2018). Toxicities were manageable and were tolerated in the outpatient clinic with weekly adjustment of dosages according to toxicity. On the basis of the results, PCE can be considered for a first line treatment in patients with recurrent and/or metastatic SCCHN (Grade C1) in the Japanese Society of Medical Oncology (JSMO) guidance of anticancer drugs for head and neck cancer.
2. Biomarker analysis
To establish a prognostic signature for locally advanced tongue squamous cell carcinoma (TSCC) patients treated with surgery, we conducted gene expression profiling of 26 clinicopathologically homogeneous advanced TSCC tissue samples using cDNA microarray as a discovery study (Enokida T et al., Oncotarget, 2017). Candidate genes were screened using clustering analysis and univariate Cox regression analysis for relapse-free survival (RFS). These were combined into a prognostic index (PI), which was validated using three public microarray datasets of tongue and oral cancer (123 patients). Some genes identified in discovery were immunohistochemically examined for protein expression in another 127 TSCC patients. In the discovery study, unsupervised hierarchical clustering analysis identified two clusters which differentiated the Kaplan-Meier curves of RFS. Thirty genes identified were combined into a dichotomous PI. In the validation cohort, classification according to the PI was associated with RFS and disease-specific survival (DSS). Among genes, positive immunohistochemical staining of cytokeratin 4 was associated with favorable prognostic values for RFS and DSS. We identified robust molecular markers that showed significant associations with prognosis in TSCC patients.
3. Retrospective analysis of management of treatment for HNC
Although gemcitabine (GEM) is thought to play a critical role in the treatment of nasopharyngeal cancer (NPC), no research to evaluate the efficacy and toxicity of GEM monotherapy has been conducted in Japan. Therefore, we retrospectively reviewed eight nasopharyngeal carcinoma patients treated with GEM monotherapy at our institute between May 2015 and August 2016 (Enokida T et al., Int J Clin Oncol, 2017). All patients were administered GEM 800-1,000 mg/m2 on days 1, 8, and 15, repeated every four weeks. One patient had a complete response and one had a partial response, giving an overall response rate of 25%; four patients (50%) had stable disease and two (25%) experienced disease progression. Toxicities were manageable and were tolerated with no treatment-related deaths. Our findings suggest that GEM monotherapy is well tolerated and has potential as an active agent in Japanese patients with recurrent/metastatic NPC who had been heavily pretreated.
Clinical trials
The following investigator-initiated clinical trials are ongoing: 1) a feasibility study of a combination with paclitaxel, carboplatin, and cetuximab (PCE) as induction chemotherapy for unresectable locally advanced SCCHN, 2) JCOG1008: a randomized phase II/III trial of postoperative CRT comparing weekly CDDP plus RT with three weekly CDDP plus RT in high risk patients with SCCHN, 3) a phase II study of lenvatinib for anaplastic thyroid cancer, and 4) a cohort study exploring the effect of lenvatinib on differentiated thyroid cancer.
To facilitate the timely approval of new drugs and eliminate the drug lag, we have also participated in the global phase trials including immune checkpoint inhibitors. Phase I study of photoimmunotherapy is also ongoing.
Education
We educate not only medical staff in our institute but also outside our institute by conducting the following education programs: 1) Seminar of Japanese Society of Supportive Care for Patients with HNC and 2) Preceptorship in HNC. Furthermore, our department is accepting trainees all the time.
Future prospects
We hope that ongoing or planned clinical trials will change the standard of care for HNC and our biomarker analysis will lead to the development of new treatment strategies. Our education programs will increase the number of medical oncologists who take charge of treatment for HNC, leading to improving patients' quality of life.
List of papers published in January 2017 - March 2018
Journal
1. Ikegawa K, Suzuki S, Nomura H, Enokida T, Yamazaki T, Okano S, Endo K, Saito S, Yamaguchi M, Tahara M. Retrospective analysis of premedication, glucocorticosteroids, and H1-antihistamines for preventing infusion reactions associated with cetuximab treatment of patients with head and neck cancer. J Int Med Res, 45:1378-1385, 2017
2. Tahara M, Schlumberger M, Elisei R, Habra MA, Kiyota N, Paschke R, Dutcus CE, Hihara T, McGrath S, Matijevic M, Kadowaki T, Funahashi Y, Sherman SI. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer, 75:213-221, 2017
3. Enokida T, Uozumi S, Fujisawa T, Ueda Y, Okano S, Tahara M. Gemcitabine monotherapy in patients with heavily treated nasopharyngeal cancer: a case series. Int J Clin Oncol, 22:1009-1014, 2017
4. Harrington KJ, Ferris RL, Blumenschein G Jr, Colevas AD, Fayette J, Licitra L, Kasper S, Even C, Vokes EE, Worden F, Saba NF, Kiyota N, Haddad R, Tahara M, Grunwald V, Shaw JW, Monga M, Lynch M, Taylor F, DeRosa M, Morrissey L, Cocks K, Gillison ML, Guigay J. Nivolumab versus standard, single-agent therapy of investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol, 18:1104-1115, 2017
5. Enokida T, Nishikawa H. Regulatory T cells, as a target in anticancer immunotherapy. Immunotherapy, 9:623-627, 2017
6. Kiyota N, Robinson B, Shah M, Hoff AO, Taylor MH, Li D, Dutcus CE, Lee EK, Kim SB, Tahara M. Defining Radioiodine-Refractory Differentiated Thyroid Cancer: Efficacy and Safety of Lenvatinib by Radioiodine-Refractory Criteria in the SELECT Trial. Thyroid, 27:1135-1141, 2017
7. Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, Sasaki T, Suzuki T, Fujino K, Dutcus CE, Takahashi S. Lenvatinib for Anaplastic Thyroid Cancer. Frontiers in oncology, 7:25, 2017
8. Nakamura N, Zenda S, Tahara M, Okano S, Hayashi R, Hojo H, Hotta K, Kito S, Motegi A, Arahira S, Tachibana H, Akimoto T. Proton beam therapy for olfactory neuroblastoma. Radiother Oncol, 122:368-372, 2017
9. Yokota T, Ogawa T, Takahashi S, Okami K, Fujii T, Tanaka K, Iwae S, Ota I, Ueda T, Monden N, Matsuura K, Kojima H, Ueda S, Sasaki K, Fujimoto Y, Hasegawa Y, Beppu T, Nishimori H, Hirano S, Naka Y, Matsushima Y, Fujii M, Tahara M. Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study. BMC Cancer, 17:314, 2017
10. Tuttle RM, Brose MS, Grande E, Kim SW, Tahara M, Sabra MM. Novel concepts for initiating multitargeted kinase inhibitors in radioactive iodine refractory differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab, 31:295-305, 2017
11. Yamazaki T, Tahara M, Enokida T, Wakasugi T, Arahira S, Zenda S, Motegi A, Akimoto T, Yoshisue K. Pharmacokinetics of initial full and subsequent reduced doses of S-1 in patients with locally advanced head and neck cancer-effect of renal insufficiency. Jpn J Clin Oncol, 47:407-412, 2017
12. Kiyota N, Hasegawa Y, Takahashi S, Yokota T, Yen CJ, Iwae S, Shimizu Y, Hong RL, Goto M, Kang JH, Sum Kenneth Li W, Ferris RL, Gillison M, Namba Y, Monga M, Lynch M, Tahara M. A randomized, open-label, Phase III clinical trial of nivolumab vs. therapy of investigator's choice in recurrent squamous cell carcinoma of the head and neck: A subanalysis of Asian patients versus the global population in checkmate 141. Oral oncology, 73:138-146, 2017
13. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, Castro A, Licitra L, Adelstein D, Vermorken JB. Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemoradiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist, 22:1056-1066, 2017
14. Argiris A, Harrington KJ, Tahara M, Schulten J, Chomette P, Ferreira Castro A, Licitra L. Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Frontiers in oncology, 7:72, 2017
15. Suzuki S, Abbott R, Sakurai H, Kawasumi K, Johnson PE, Tahara M, Yamaguchi M, Saito S, Yee GC, Endo K. Evaluation of community pharmacist ability to ensure the safe use of oral anticancer agents: a nationwide survey in Japan. Jpn J Clin Oncol, 47:413-421, 2017
16. Cohen EEW, Licitra LF, Burtness B, Fayette J, Gauler T, Clement PM, Grau JJ, Del Campo JM, Mailliez A, Haddad RI, Vermorken JB, Tahara M, Guigay J, Geoffrois L, Merlano MC, Dupuis N, Kramer N, Cong XJ, Gibson N, Solca F, Ehrnrooth E, Machiels JH. Biomarkers predict enhanced clinical outcomes with afatinib versus methotrexate in patients with second-line recurrent and/or metastatic head and neck cancer. Ann Oncol, 28:2526-2532, 2017
17. Enokida T, Fujii S, Takahashi M, Higuchi Y, Nomura S, Wakasugi T, Yamazaki T, Hayashi R, Ohtsu A, Tahara M. Gene expression profiling to predict recurrence of advanced squamous cell carcinoma of the tongue: discovery and external validation. Oncotarget, 8:61786-61799, 2017
18. Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S, Onoe T, Homma A, Taguchi J, Suzuki M, Minato K, Yane K, Ueda S, Hara H, Saijo K, Yamanaka T. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol, 29:1004-1009, 2018
19. Tahara M, Muro K, Hasegawa Y, Chung HC, Lin CC, Keam B, Takahashi K, Cheng JD, Bang YJ. Pembrolizumab in Asia-Pacific patients with advanced head and neck squamous cell carcinoma: Analyses from KEYNOTE-012. Cancer Sci, 109:771-776, 2018
20. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, Adelstein D, Vermorken JB. Altered fractionation radiotherapy combined with concurrent low-dose or high-dose cisplatin in head and neck cancer: A systematic review of literature and meta-analysis. Oral Oncol, 76:52-60, 2018
