Annual Report 2017
Department of Pathology and Clinical Laboratories / Clinical Laboratories
Takeshi Kuwata, Masato Sugano, Atsushi Ochiai, Genichiro Ishii, Satoshi Fujii, Motohiro Kojima, Hiroshi Nakamura, Akiko Nagatsuma, Eiichi Yoshikawa, Shigeyuki Hasuo, Shigehisa Yoshida, Masahiro Karibe, Miki Goto, Seiji Iwasaki, Masaki Takeda, Noriaki Sato, Kumi Horiuchi, Hiromi Kimura, Yasuharu Hashimoto, Yukihiro Okano, Mari Hisano, Mitsunori Tajima, Megumi Hasegawa, Mika Narikiyo, Yota Ikegami, Mika Sasanuma, Aya Koike, Takuya Yamaguchi, Takuya Aiba, Keiko Nakai, Ayumi Setsuta, Mayumi Kasai, Ayumi Nakanishi, Ayumi Iwaya, Misato Nojiri, Tomoko Ikeda, Akira Miyaura, Yuki Takahashi, Masahiro Inoue, Yasuko Yoshihara, Izumi Suzuki, Kazumi Yamaguchi, Sayuri Shibayama, Midori Kawamura, Ikuko Takahashi, Saki Nakamura, Kazuki Motohashi, Chihiro Kodama, Chihiro Kodama, Yukina Fukuda, Keiko Arai, Sakie Nakahata, Chiharu Eda, Eriko Iwamoto, Michiteru Yamagishi, Nagisa Bono, Kasumi Tamura, Tomomi Sekine, Rie Kuroiwa, Yuki Soeda, Masayuki Ito, Mitiko Iida, Natsuki Nagamine, Takuya Hanazawa, Sayaka Sekine, Mayu Sasagawa, Hiroshi Matsui, Osamu Takahashi, Narumi Yokota, Yuriko Hayashi, Emiko Yoshikawa, Megumi Imamiya, Yuko Iwata, Yoshiko Otake, Megumi Yamaguchi, Miwa Yamada, Noriko Sato, Mariko Kinoshita
Introduction
The Department of Pathology and Clinical Laboratories (DPCL) has two divisions; the Pathology Division (PD) and the Clinical Laboratory Division (CLD). Both divisions play a fundamental role in routine hospital services and support research activities at the National Cancer Center Hospital East (NCCHE).
In 2012, the DPCL received the accreditation of ISO 15189 : 2007, ensuring quality control and quality assurance of testing, including the one for clinical trials, successfully transited to the newest version (ISO 15189 : 2012) in 2014, and firstly updated in 2017.
Our team and what we do
Pathology Division (PD) : All the pathologists engage in surgical pathology and research for cancer biology or development of new drugs. The number of samples examined at our department in 2017 is listed in Table 1.
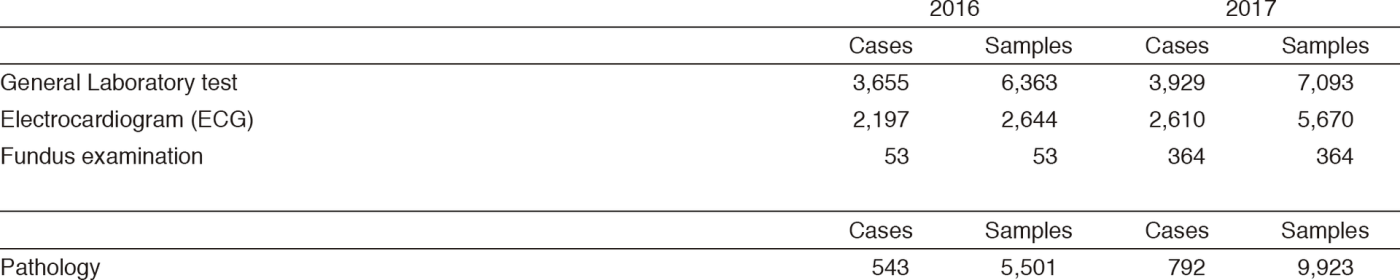
Clinical Laboratory Division (CLD) : This division consists of eight sections; 1) General Laboratory Medicine, 2) Hematology, 3) Biochemistry and Immunology, 4) Physiological Function Testing, 5) Bacteriology, 6) Blood Transfusion, 7) Pathology and Genetic Testing, and 8) Supporting Laboratory Testing in Clinical Studies. The numbers of testing performed in each division are listed in Tables 2 and 3.
The total number of tests performed in the DPCL in 2017 increased by 9.5% compared with the previous year.
Table 1. Number of pathology and cytology samples examined at the Pathology Division in 2017 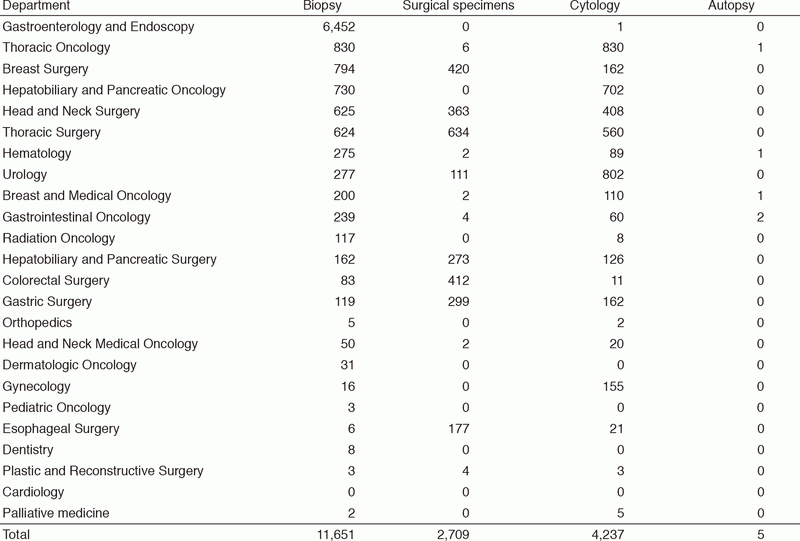
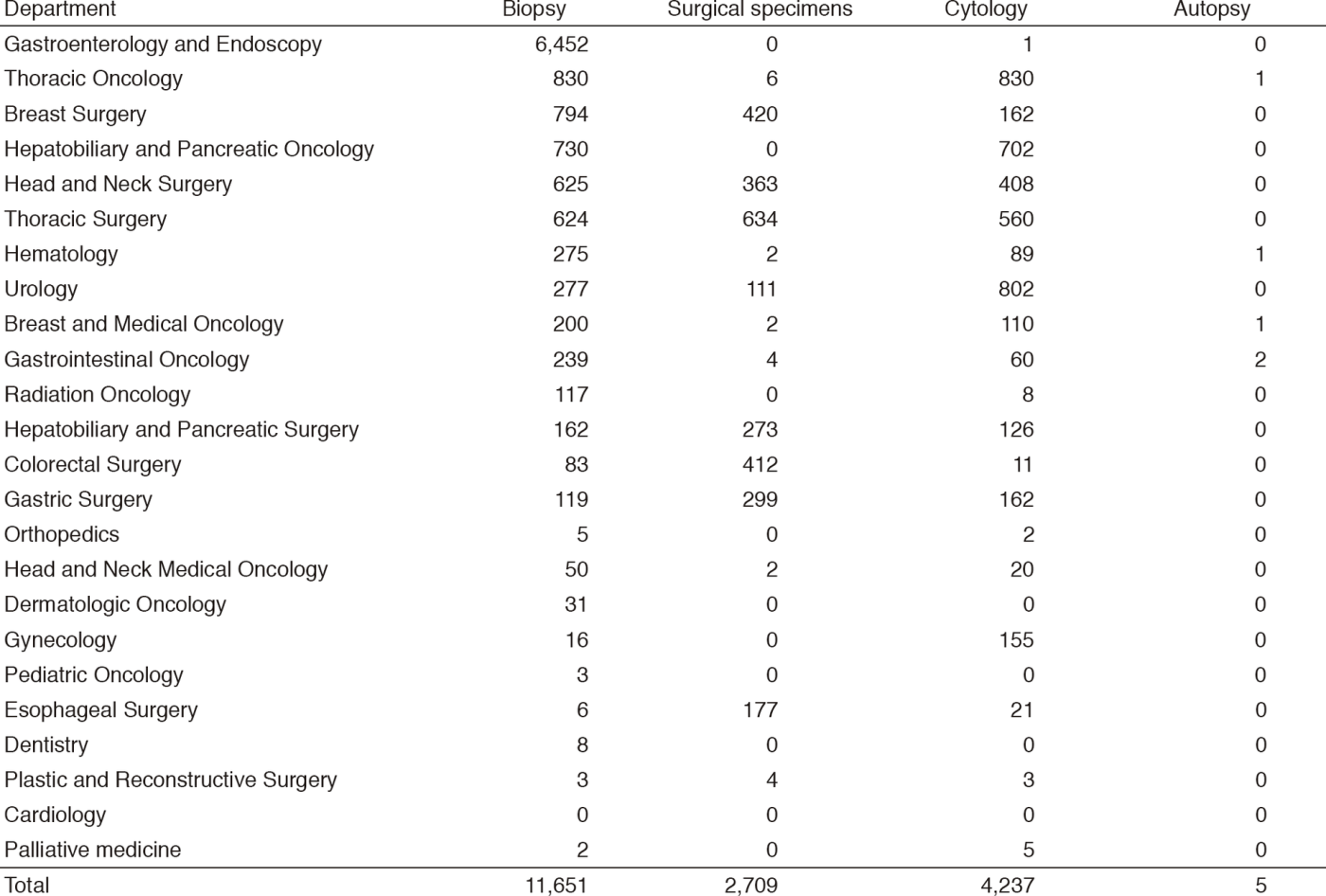
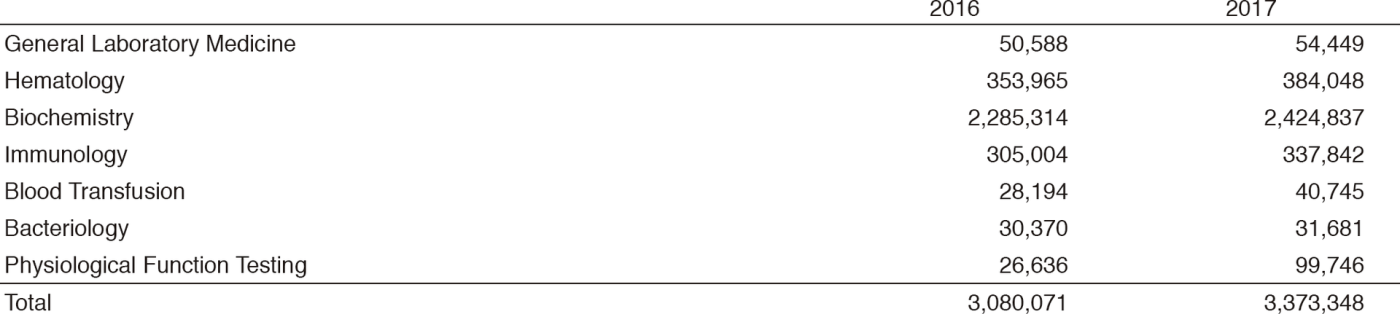
Table 2. Number of laboratory tests examined at the Clinical Laboratory Division in 2016 & 2017
Research activities
All of the pathologists were involved in research activities at the Exploratory Oncology Research and Clinical Trial Center (EPOC). All the technologists working in our department are also highly motivated to develop advanced diagnostic technologies and various results are presented in several meetings.
Clinical trials
The Section for Supporting Laboratory Testing in Clinical Studies, coordinating with the Section for Pathology and the Section for Physiological Function Testing, reinforces quality control and quality assurance for clinical tests performed in clinical trials at the NCCHE. Practically, the CLD participated in all of the clinical trials operated at the NCCHE by providing laboratory data.
Education
The staff of the CLD regularly conduct study sessions or lecture meetings in order to improve their expertise and testing technique based on the requirement of ISO 15189.
Clinicopathological conferences are held regularly with each clinical department/section. In the PD, conference-style training sessions are open weekly for residents.
Future prospects
Pathological diagnosis and laboratory tests play a fundamental role not just in routine hospital works but also in medical research. Additionally, a genetic testing section will be managed for implementation of genomic cancer medicine. As an ISO15189-certified clinical laboratory, the DPCL will be continuously involved in investigating new diagnostic technologies, developing new drugs, and conducting translational / clinical research in the NCCHE.
List of papers published in January 2017 - March 2018
Journal
1. Higaki E, Yanagi S, Gotohda N, Kinoshita T, Kuwata T, Nagino M, Ochiai A, Fujii S. Intraoperative peritoneal lavage cytology offers prognostic significance for gastric cancer patients with curative resection. Cancer Sci, 108:978-986, 2017
2. Ichikawa T, Saruwatari K, Mimaki S, Sugano M, Aokage K, Kojima M, Hishida T, Fujii S, Yoshida J, Kuwata T, Ochiai A, Suzuki K, Tsuboi M, Goto K, Tsuchihara K, Ishii G. Immunohistochemical and genetic characteristics of lung cancer mimicking organizing pneumonia. Lung Cancer, 113:134-139, 2017
3. Neri S, Miyashita T, Hashimoto H, Suda Y, Ishibashi M, Kii H, Watanabe H, Kuwata T, Tsuboi M, Goto K, Menju T, Sonobe M, Date H, Ochiai A, Ishii G. Fibroblast-led cancer cell invasion is activated by epithelial-mesenchymal transition through platelet-derived growth factor BB secretion of lung adenocarcinoma. Cancer Lett, 395:20-30, 2017
4. Ikemura S, Aramaki N, Fujii S, Kirita K, Umemura S, Matsumoto S, Yoh K, Niho S, Ohmatsu H, Kuwata T, Kojima M, Ochiai A, Betsuyaku T, Tsuboi M, Goto K, Ishii G. Changes in the tumor microenvironment during lymphatic metastasis of lung squamous cell carcinoma. Cancer Sci, 108:136-142, 2017
5. Shimizu K, Kirita K, Aokage K, Kojima M, Hishida T, Kuwata T, Fujii S, Ochiai A, Funai K, Yoshida J, Tsuboi M, Ishii G. Clinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinoma. J Cancer Res Clin Oncol, 143:321-328, 2017
6. Shitara K, Doi T, Nagano O, Fukutani M, Hasegawa H, Nomura S, Sato A, Kuwata T, Asai K, Einaga Y, Tsuchihashi K, Suina K, Maeda Y, Saya H, Ohtsu A. Phase 1 study of sulfasalazine and cisplatin for patients with CD44v-positive gastric cancer refractory to cisplatin (EPOC1407). Gastric cancer, 20:1004-1009, 2017
7. Shitara K, Doi T, Nagano O, Imamura CK, Ozeki T, Ishii Y, Tsuchihashi K, Takahashi S, Nakajima TE, Hironaka S, Fukutani M, Hasegawa H, Nomura S, Sato A, Einaga Y, Kuwata T, Saya H, Ohtsu A. Dose-escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer, 20:341-349, 2017
8. Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer, 20:407-415, 2017
9. Goto M, Naito M, Saruwatari K, Hisakane K, Kojima M, Fujii S, Kuwata T, Ochiai A, Nomura S, Aokage K, Hishida T, Yoshida J, Yokoi K, Tsuboi M, Ishii G. The ratio of cancer cells to stroma after induction therapy in the treatment of non-small cell lung cancer. J Cancer Res Clin Oncol, 143:215-223, 2017