Annual Report 2017
Clinical Research Support Office
·Research Management DivisionAkihiro Sato - Project Management / Regulatory Affairs Management Section
Nozomu Fusea
- Clinical Trial Management Section
Miki Fukutani, Hiromi Ono, Sakiko Kuroda, Rena Sato, Hitomi Tamura, Masako Nakamoto, Makoto Fukui, Yuichi Mikamoto, Shinobu Iida, Michiko Suzuki, Sachiko Sakamoto, Koji Takahashi, Maiko Takakusa, Hikari Sima
- Data Management Section
Hidekazu Tsuboki, Natsuko Iwasaki, Ayako Sugama , Kyouko Tokita, Kaori Tobayama, Tsukiko Higuchi, Nami Hirano, Seiko Matsuda, Akiomi Yoshida, Yuya Suga, Michio Ohono, Kei Ikeno, Kazuko Tsukamoto
- Biostatistics Section
Shogo Nomura, Masashi Wakabayashi
- Pharmacovigilance Section
Masato Yonemura, Noriko Fujishiro, Kanae Kurashige
- Information Technology Management Section
Yoshihiro Aoyagi
Katsuya Tsuchihara
- Biobank Translational Research Support Section
Wataru Okamoto
- Biobank Research Concierge
Akiko Nakayama
- Translational Research Support
Izumi Miki, Yasutoshi Sakamoto
- Research Sample Management Support
Yasuko Tada
- Seeds Development Support Section
Katsuji Aikawa
Takayuki Yoshino, Masafumi Ikeda, Yoichi Naito, Kiyotaka Yoh, Takashi Kojima, Nobuaki Matsubara, Yasutoshi Kuboki, Takahiro Sakai, Yukie Kimura, Miyuki Hara, Yuko Ito, Ikumi Tamaki, Mayumi Shirase, Keiko Yamamoto, Chiyo Ito, Koko Komata, Aya Shinbu, Chiharu Hirano, Kiyoko Adachi, Keiko Abe, Rie Taniguchi, Kyoko Tsuyuki, Hiromi Motoyanagi, Kazumi Yamaguchi, Yasuko Yoshihara, Kyoko Uehara, Noriko Noda, Yoshimi Fujiki, Junko Tsukada, Megumi Hodota, Rikako Tanaka, Megumi Futamura, Masami Sasaki, Mizuho Ibaraki, Aki Hashimoto, Mikina Takiguchi, Tomoko Matsumura, Atsuko Katagiri, Haruna Ariyoshi, Hikaru Matsuki, Yasuko Nishikubo, Masumi Kudo, Yoshimi Izumi, Minako Suzuki, Mayumi Nagino, Fumiko Kase, Tomoko Watanabe, Azusa Funaba, Yoko Matsuda
Introduct ion
The Clinical Research Support Office supports clinical trial programs conducted in the National Cancer Center Hospital East (NCCHE).
Our team and what we do
1. Research Management Division
We support clinical trial programs through the clinical datacenter, study management, site visit monitoring, safety information, and bio statistics.
2. Translational Research Management Division
We support the translational research including seeds development, biomarker discovery research, and the development of in vitro diagnostics and companion diagnostics.
3. Clinical Research Coordinating Division
This division mainly focuses on first-in-human clinical trials as well as investigator initiated innovative clinical trials for unmet medical needs.
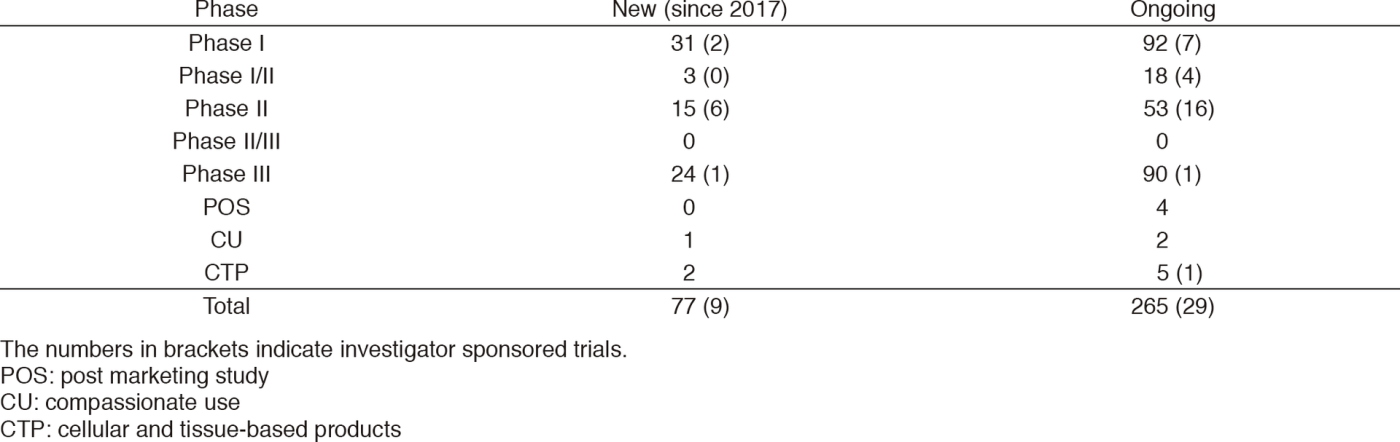
Clinical trials
1. Research Management Division
In 2017, six IND (Investigational New Drug) trials started their enrollment.
2. Translational Research Management Division
We have supported Nationwide Genome Screening Project: SCRUM-Japan since 2015, which is planned to collect about 10,000 patients' clinical and genomic data by the end of 2018.
We started the study support regarding ctDNA analysis (liquid biopsy) from 2017.
Education
- On-the-job training for new staff
- Supporting educational programs for clinical trial methodology and GCP (Good Clinical Practice) in the National Cancer Center (NCC)
- Clinical trial education programs for other institutions' investigators
Future prospects
We will enhance capability of clinical trial support.