Annual Report 2017
Department of Breast and Medical Oncology
Kenji Tamura, Kan Yonemori, Emi Noguchi, Akihiko Shimomura, Kazuki Sudo, Maki Tanioka, Hitomi Okuma, Tadaaki Nishikawa, Takuji Seo, Yohei Ohtake, Yuki Kojima and Yasuhiro Fujiwara.
Introduction
The Department of Breast and Medical Oncology provides the most effective treatment by the use of chemotherapy, and works on the establishment of new standard of care for adult malignancies including breast cancer, gynecologic cancer, soft-tissue sarcoma, extragonadal germ cell tumors, cancer of unknown primary, and other rare types of solid tumors.
We envision becoming a leading medical oncology department, which makes a difference in cancer care in Japan and in the world. Our mission is to provide patient-centered, state-of-the-art medical care to cancer patients, to develop new effective cancer treatment through clinical and translational research, and to nurture medical oncologists. An evidence-based, research-oriented, and multi-disciplinary approach is the core value of our practice.
Our team and what we do
1. Setup
Our department consists of eight full-time attending physicians, four chief residents (fellows), and two to three clinical residents. We also provide educational opportunities to short-term (a half year) residents. Full-time attending physicians are on duty at the outpatient clinic two to three days per week. The inpatient management is undertaken by clinical teams consisting of attending physicians and residents. A Grand Round is scheduled for every Wednesday and Friday.
2. Performance
There were 1,474 first visits of new patients in 2017 (Table 1). 39.7% of new patients are breast cancer patients, 17.4% are gynecological cancer patients, 20.8% are patients with cancer of primary unknown, and 10% are patients with soft-tissue sarcoma in the first visits. We provided 541 second opinions in 2017.
Table 1. 1st Visiting Patients to the Department of Breast and medical Oncology (January 2017 - March 2018)

3. Conference
A one-hour briefing medical conference is held every morning to discuss the evidence-based care for individual patients. A conference on phase I is held on Monday, journal club on Wednesday, clinical trial conference on Thursday, and the weekend and outpatient follow-up conference on Friday. Multidisciplinary case conferences with diagnostic radiologists, surgeons, and pathologists are held with members of the Departments of Breast Surgery, Gynecology, Musculoskeletal Oncology and Rehabilitation, Radiation Oncology, and the Pathology Division, once or twice (Breast) per week, respectively.
The monthly breast cancer conference is held with the participation of multidisciplinary specialists to discuss recent topics in breast oncology and to update institutional treatment guidelines.
Research activities
Our research interest extends across wide range of topics related to treatment and clinical program development. A lot of our researches are secured by public and consignment research grants. In 2017, we conducted many research programs as the primary investigator and participated in additional programs as the co-investigator in research programs secured by competitive public research funds. We published 43 international manuscripts, focusing on early phase anti-cancer drug development, molecular imaging, translational research, novel chemotherapy against sarcoma and ovarian cancer, novel biomarkers to predict efficacy and adverse events of anti-cancer drugs, and other basic research. We value cancer survivorship as a research theme in order to develop a patient-centered comprehensive care program.
Clinical trials
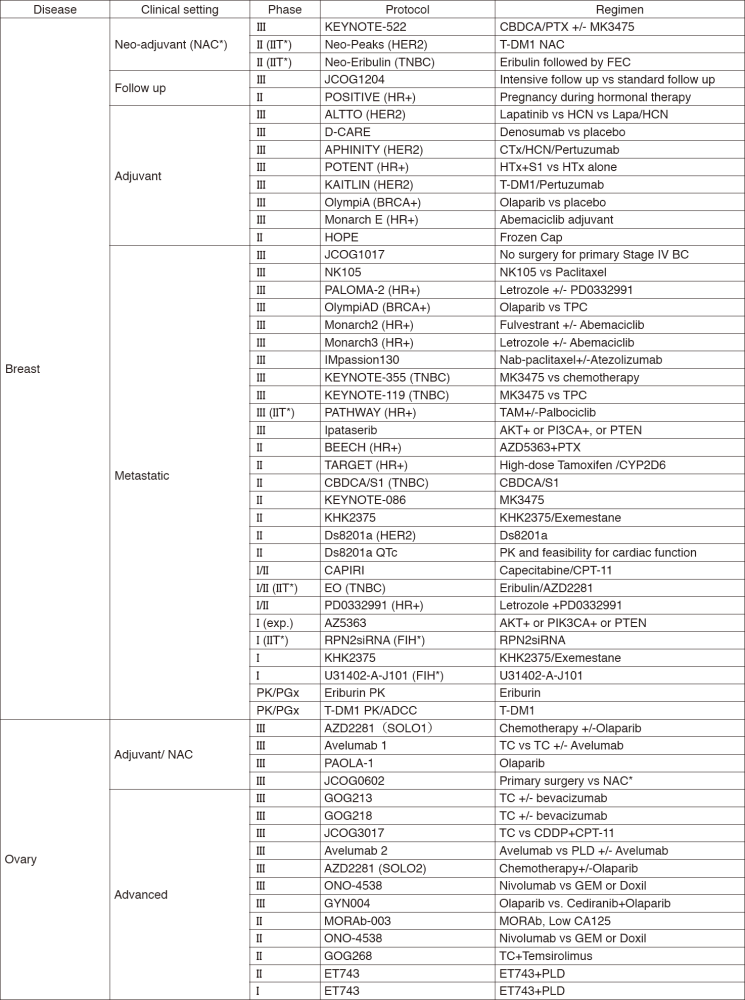
In 2017, we actively enrolled patients in phase I studies (including first in human or global) as well as domestic and international phase II and III studies (Table 2). Of note, we launched a pharmacokinetic and dose-finding study of eribulin/olaparib, a phase II study of eribulin in neoadjuvant setting for patients with triple negative breast cancer, a phase III study of tamoxifen with/without palbociclib, Ds8201a for uterine cancer sarcoma and phase I of RPN2 (first in human) as investigator-initiated clinical trials (IIT in Table 2). New molecular imaging studies are launched in cooperation with the National Cancer Center, Research Institute (NCCRI). We also conducted many types of translational research (TR) to find novel biomarkers.
Education
We provide rich educational opportunities to both residents and chief residents through clinical experience as well as research activities. Residents are encouraged to make presentations at local and national conferences. We vigorously support basic, clinical, or translational researches conducted by postdoctoral researchers.
Future prospects
We will continue to establish new standard treatments and propose a near-future model of clinical management of adult solid tumors, including breast cancer and gynecologic cancer. Moreover, we aim to build a comprehensive program, which includes a tumor registry, translational research, clinical trials, and patient care in rare adult tumors based on our rich clinical experience. We would also like to improve the efficiency of anti-cancer drug development by coordinating basic and translational research in early-phase clinical trials.
Table 2. Active Clinical Trials (Januay 2017 - March 2018)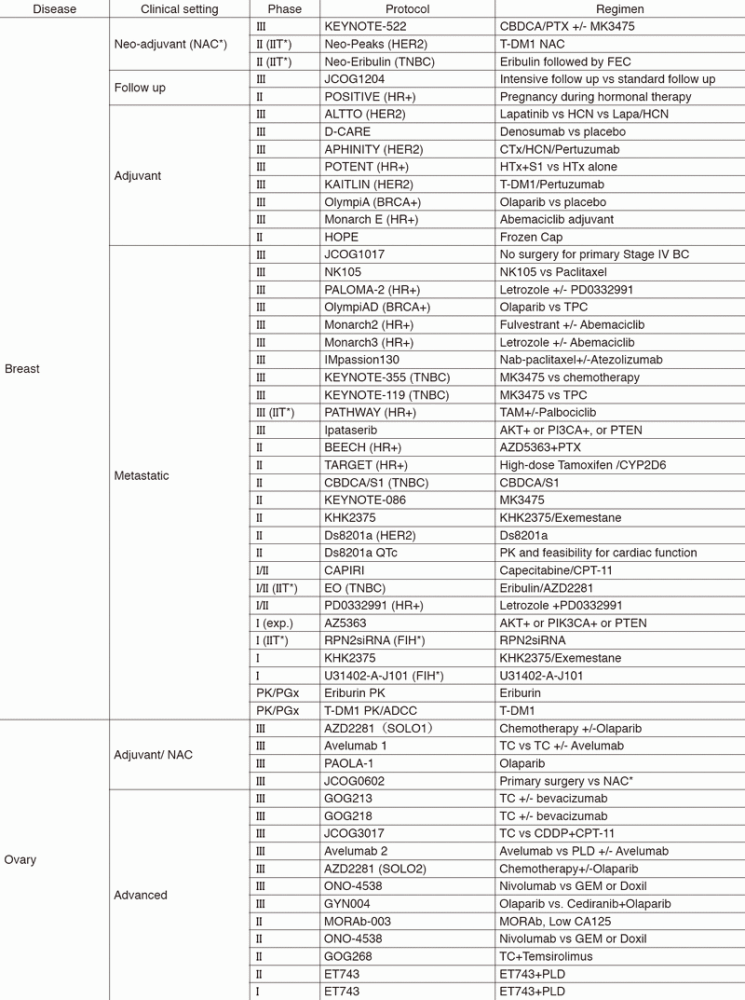
List of papers published in January 2017 - March 2018
Journal
1.Ebata T, Yunokawa M, Yoshida H, Bun S, Shimoi T, Shimomura A, Kodaira M, Yonemori K, Shimizu C, Fujiwara Y, Kato T, Tamura K. The Prognostic Impact of the Pathological Response to Neoadjuvant Dose-Dense Therapy for Ovarian Carcinoma. Int J Gynecol Cancer, 27:1850-1855, 2017
2.Tamura K, Inoue K, Masuda N, Takao S, Kashiwaba M, Tokuda Y, Iwata H, Yamamoto N, Aogi K, Saeki T, Nakayama T, Sato N, Toyama T, Ishida T, Arioka H, Saito M, Ohno S, Yamauchi H, Yamada K, Watanabe J, Ishiguro H, Fujiwara Y. Randomized phase II study of nab-paclitaxel as first-line chemotherapy in patients with HER2-negative metastatic breast cancer. Cancer Sci, 108:987-994, 2017
3.Iwamoto N, Shimomura A, Tamura K, Hamada A, Shimada T. LC-MS bioanalysis of Trastuzumab and released emtansine using nano-surface and molecular-orientation limited (nSMOL) proteolysis and liquid-liquid partition in plasma of Trastuzumab emtansine-treated breast cancer patients. J Pharm Biomed Anal, 145:33-39, 2017
4.Nishikawa T, Matsumoto K, Tamura K, Yoshida H, Imai Y, Miyasaka A, Onoe T, Yamaguchi S, Shimizu C, Yonemori K, Shimoi T, Yunokawa M, Xiong H, Nuthalapati S, Hashiba H, Kiriyama T, Leahy T, Komarnitsky P, Fujiwara K. Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors. Cancer Sci, 108:1834-1842, 2017
5.Shimomura A, Kondo S, Kobayashi N, Iwasa S, Kitano S, Tamura K, Fujiwara Y, Yamamoto N. Do all patients in the phase I oncology trials need to be hospitalized? Domestic but outstanding issues for globalization of drug development in Japan. Int J Clin Oncol, 22:780-785, 2017
6.Honma Y, Hokamura N, Nagashima K, Sudo K, Shoji H, Iwasa S, Takashima A, Kato K, Hamaguchi T, Boku N, Umezawa R, Ito Y, Itami J, Koyanagi K, Igaki H, Tachimori Y. Clinical Outcomes of Resectable Esophageal Cancer with Supraclavicular Lymph Node Metastases Treated with Curative Intent. Anticancer Res, 37:3741-3749, 2017
7.Fujiwara Y, Ono S. Regulatory Review of New Therapeutic Agents. N Engl J Med, 376:2598, 2017
8.Makise N, Yoshida A, Komiyama M, Nakatani F, Yonemori K, Kawai A, Fukayama M, Hiraoka N. Dedifferentiated Liposarcoma With Epithelioid/Epithelial Features. Am J Surg Pathol, 41:1523-1531, 2017
9.Iizumi S, Shimoi T, Tsushita N, Bun S, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Efficacy and safety of eribulin in patients with locally advanced or metastatic breast cancer not meeting trial eligibility criteria: a retrospective study. BMC Cancer, 17:819, 2017
10.Yamamoto N, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Tanabe Y, Horiike A, Yanagitani N, Kitazono S, Inatani M, Tanaka J, Nishio M. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs, 35:37-46, 2017
11.Kitano A, Ono M, Yoshida M, Noguchi E, Shimomura A, Shimoi T, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Fujiwara Y, Tsuda H, Tamura K. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO open, 2:e000150, 2017
12.Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, Shimomura A, Yonemori K, Shimizu C, Hamada A, Kanayama Y, Watanabe Y, Fujiwara Y, Tamura K. Visualization of HER2-specific breast cancer intratumoral heterogeneity using 64Cu-DOTA-trastuzumab PET. Eur J Nucl Med Mol Imaging, 44:2146-2147, 2017
13.Okuma HS, Yonemori K. BRCA Gene Mutations and Poly(ADP-Ribose) Polymerase Inhibitors in Triple-Negative Breast Cancer. Advances in experimental medicine and biology, 1026:271-286, 2017
14.Takeuchi E, Kato M, Wada S, Yoshida S, Shimizu C, Miyoshi Y. Physicians' practice of discussing fertility preservation with cancer patients and the associated attitudes and barriers. Support Care Cancer, 25:1079-1085, 2017
15.Yonemori KM, Ennis T, Novotny R, Fialkowski MK, Ettienne R, Wilkens LR, Leon Guerrero RT, Bersamin A, Coleman P, Li F, Boushey CJ. Collecting wrappers, labels, and packages to enhance accuracy of food records among children 2-8 years in the Pacific region: Children's Healthy Living Program (CHL). Journal of food composition and analysis, 64:112-118, 2017
16.Tanabe Y, Shimizu C, Hamada A, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yamamoto H, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y. Paclitaxel-induced sensory peripheral neuropathy is associated with an ABCB1 single nucleotide polymorphism and older age in Japanese. Cancer Chemother Pharmacol, 79:1179-1186, 2017
17.Miyoshi Y, Yorifuji T, Horikawa R, Takahashi I, Nagasaki K, Ishiguro H, Fujiwara I, Ito J, Oba M, Fujisaki H, Kato M, Shimizu C, Kato T, Matsumoto K, Sago H, Takimoto T, Okada H, Suzuki N, Yokoya S, Ogata T, Ozono K. Childbirth and fertility preservation in childhood and adolescent cancer patients: a second national survey of Japanese pediatric endocrinologists. Clinical pediatric endocrinology : case reports and clinical investigations, 26:81-88, 2017
18.Tanabe Y, Tsuda H, Yoshida M, Yunokawa M, Yonemori K, Shimizu C, Yamamoto S, Kinoshita T, Fujiwara Y, Tamura K. Pathological features of triple-negative breast cancers that showed progressive disease during neoadjuvant chemotherapy. Cancer Sci, 108:1520-1529, 2017
19.Hironaka-Mitsuhashi A, Matsuzaki J, Takahashi RU, Yoshida M, Nezu Y, Yamamoto Y, Shiino S, Kinoshita T, Ushijima T, Hiraoka N, Shimizu C, Tamura K, Ochiya T. A tissue microRNA signature that predicts the prognosis of breast cancer in young women. PLoS One, 12:e0187638, 2017
20.Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, Shimomura A, Yunokawa M, Yonemori K, Shimizu C, Hamada A, Kanayama Y, Watanabe Y, Fujiwara Y, Tamura K. 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann Oncol, 28:2028-2029, 2017
21.Sasada S, Ushirozawa N, Kobayashi N, Fujiwara Y, Tamura K, Yamamoto N. Surveillance of protocol deviations in Japanese oncology registration trials: a single institute experience. Invest New Drugs, 35:392-396, 2017
22.Ebata T, Shimoi T, Ishiwata T, Iwasawa S, Bun S, Yunokawa M, Yonemori K, Takiguchi Y, Tamura K. Amrubicin Monotherapy for Patients with Platinum-Pretreated Non-Gastrointestinal Non-Pancreatic Extrapulmonary Neuroendocrine Carcinoma. Oncology, 93:177-182, 2017
23.Iizumi S, Shimoi T, Nishikawa T, Kitano A, Sasada S, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Prolonged Hypocalcemia Following a Single Dose of Denosumab for Diffuse Bone Metastasis of Gastric Cancer after Total Gastrectomy. Intern Med, 56:2879-2882, 2017
24.Nishiumi S, Kobayashi T, Kawana S, Unno Y, Sakai T, Okamoto K, Yamada Y, Sudo K, Yamaji T, Saito Y, Kanemitsu Y, Okita NT, Saito H, Tsugane S, Azuma T, Ojima N, Yoshida M. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget, 8:17115-17126, 2017
25.Yunokawa M, Yoshida H, Watanabe R, Noguchi E, Shimomura A, Shimoi T, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Allred score is a promising predictor of prognosis and medroxyprogesterone acetate efficacy in patients with endometrial cancer. Cancer Chemother Pharmacol, 80:127-134, 2017
26.Kawai A, Yonemori K, Takahashi S, Araki N, Ueda T. Systemic Therapy for Soft Tissue Sarcoma: Proposals for the Optimal Use of Pazopanib, Trabectedin, and Eribulin. Adv Ther, 34:1556-1571, 2017
27.Tsubata Y, Hayashi M, Tanino R, Aikawa H, Ohuchi M, Tamura K, Fujiwara Y, Isobe T, Hamada A. Evaluation of the heterogeneous tissue distribution of erlotinib in lung cancer using matrix-assisted laser desorption ionization mass spectrometry imaging. Sci Rep, 7:12622, 2017
28.Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N, Kitano A, Jikoh T, Lee C, Fujisaki Y, Ogitani Y, Yver A, Tamura K. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol, 18:1512-1522, 2017
29.Kanke Y, Shimomura A, Saito M, Honda T, Shiraishi K, Shimada Y, Watanabe R, Yoshida H, Yoshida M, Shimizu C, Takahashi K, Totsuka H, Ogiwara H, Hirose S, Kono K, Tamura K, Okamoto A, Kinoshita T, Kato T, Kohno T. Gene aberration profile of tumors of adolescent and young adult females. Oncotarget, 9:6228-6237, 2018
30.Ajani JA, Estrella JS, Chen Q, Correa AM, Ma L, Scott AW, Jin J, Liu B, Xie M, Sudo K, Shiozaki H, Badgwell B, Weston B, Lee JH, Bhutani MS, Onodera H, Suzuki K, Suzuki A, Ding S, Hofstetter WL, Johnson RL, Bresalier RS, Song S. Galectin-3 expression is prognostic in diffuse type gastric adenocarcinoma, confers aggressive phenotype, and can be targeted by YAP1/BET inhibitors. Br J Cancer, 118:52-61, 2018
31.Shimoi T, Yoshida M, Kitamura Y, Yoshino T, Kawachi A, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Ichimura K, Fukuda T, Fujiwara Y, Tamura K. TERT promoter hotspot mutations in breast cancer. Breast Cancer, 25:292-296, 2018
32.Niikura N, Shimomura A, Fukatsu Y, Sawaki M, Ogiya R, Yasojima H, Fujisawa T, Yamamoto M, Tsuneizumi M, Kitani A, Watanabe J, Matsui A, Takahashi Y, Takashima S, Shien T, Tamura K, Saji S, Masuda N, Tokuda Y, Iwata H. Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis. Breast Cancer Res Treat, 167:81-87, 2018
Book
1. Noguchi E. Chapter 7. Companion diagnostics. In: Takiguchi Y (ed), Molecular Targeted Therapy of Lung Cancer, Springer International Publishing AG., pp 117-136, 2017
