Annual Report 2017
Department of Thoracic Oncology
Yuichiro Ohe, Noboru Yamamoto, Yutaka Fujiwara, Hidehito Horinouchi, Shintaro Kanda, Yasushi Goto, Shuji Murakami, Yuji Matsumoto, Hideaki Shiraishi, Keiko Goto, Jun Sato, Ryo Morita
Introduction
Lung cancer is the leading cause of cancer death in Japan and worldwide. The incidence of lung cancer is still increasing in Japan, especially in elderly populations. The Department of Thoracic Oncology provides care for patients with primary lung cancer, mediastinal tumors, and pleural tumors. The goals of our department are to provide the highest quality treatment and establish new effective treatments for lung cancer and other thoracic malignancies through innovative clinical and translational research. To provide assistance to our patients through multidisciplinary care, staff members of our department work closely with thoracic surgeons, radiation oncologists, pharmacists, clinical research coordinators, and psychiatrists who have expertise in these areas. Our department includes eight staff physicians. Moreover, residents and trainees from other institutions have joined the Thoracic Oncology Program.
Our team and what we do
The staff physicians attend outpatient services for thoracic diseases, and our department has approximately 60 beds in the hospital. Inpatient care is carried out by five teams. Each team consists of one staff physician and one or two residents and/or trainee doctors. Protocol and case conferences are scheduled for every Monday morning and afternoon, respectively. The journal club is scheduled for Thursday mornings.
Table 1. Number of new patients in 2017

Table 2. Type of procedures in 2017

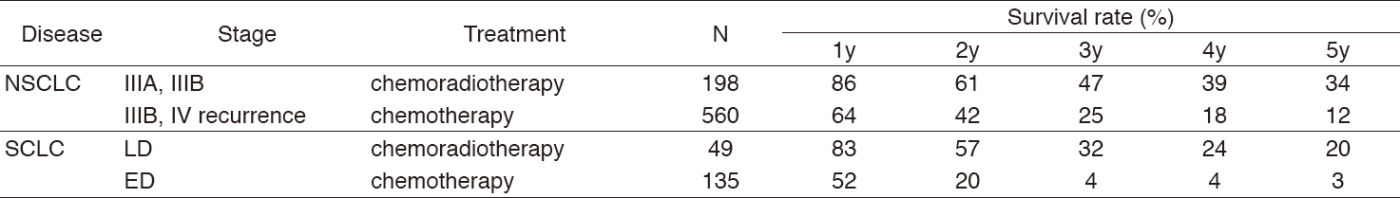
A total of 458 new patients were admitted in 2017, and the backgrounds and initial treatments of these patients are shown in Tables 1 and 2. The initial treatments were chemotherapy in 253, adjuvant chemotherapy after surgery in 51, chemoradiotherapy in 60, curative radiotherapy in seven, and supportive care including palliative radiotherapy in 34. Survival of lung cancer patients treated in 2008-2012 in our department is shown in Table 3.
Table 3. Survival of lung cancer patients treated in 2008-2012
Research activities
Research activities of the department can be classified into four categories: (1) multi-
institutional phase III studies to establish new standard treatments for lung cancer; (2) phase I and phase II studies to evaluate new anticancer drugs, (3) pharmacokinetic and pharmacodynamic (PK/PD) studies to investigate interpatient variability, optimal administration schedules, and drug-drug interactions; and (4) translational research using clinical samples from bench to bed-side or from bed-side to bench for the development of innovative treatment strategies.
Clinical trials
Our department is currently conducting and participating in multi-institutional phase III studies to establish new standard treatments for lung cancer such as the Japan Clinical Oncology Group (JCOG) trials and global trials conducted by pharmaceutical companies. Three JCOG phase III studies, JCOG1201 for elderly ED-SCLC, JCOG1206 for high grade neuroendocrine carcinoma, JCOG1404 (AGAIN), a phase III study for EGFR mutation positive NSCLC are ongoing. Our department is also participating in a nationwide genomic screening project of lung cancer with rare driver mutation (LC-SCRUM). Furthermore, our department carried out many clinical trials using 3rd generation EGFR-TKIs, anti-PD-1Ab, and anti-PD-L1Ab.
Education
In 2017, four chief residents, 16 residents joined our department. A monthly research conference is held to discuss about clinical and translational research conducted by young doctors.
Future prospects
The recent progression of lung cancer treatment is very rapid. Driver gene alteration targeted therapies such as EGFR-TKIs for EGFR mutation positive lung cancer, ALK inhibitors for ALK fusion gene positive lung cancer, ROS inhibitors for ROS1 fusion gene positive lung cancer, and BRAF plus MEK inhibitors for BRAF V600 positive lung cancer are already established as standard treatments. Other rare driver gene alterations such as RET fusion, MET mutation, and NTRAK fusion will be able to be good targets for treatment of lung cancer. Immunotherapy using anti-PD-1Ab and anti-PD-L1 Ab has been established as a standard second or third line treatment for NSCLC. Anti-PD-1 Ab, pembrolizumab and anti-PD-L1
Ab, atezolizumab have been established as a standard first line treatment for PD-L1 high expression NSCLC and stage III NSCLC after chemoradiotherapy, respectively. Combination of chemotherapy with immune checkpoint inhibitors and combination of immune checkpoint inhibitors with another immune checkpoint inhibitors will be a standard treatment of lung cancer in the near future. Immune checkpoint inhibitors will also be incorporated in treatments of early stage lung cancer.
List of papers published in January 2017 - March 2018
Journal
1. Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Asakura K, Nakagawa K, Sakurai H, Watanabe SI, Tsuta K, Ohe Y. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer, 108:115-120, 2017
2. Sekine I, Harada H, Yamamoto N, Wakabayashi M, Murakami H, Goto K, Nogami N, Seto T, Oshita F, Okamoto H, Tanaka H, Tamura T, Ishikura S, Ohe Y. Randomized phase II trial of weekly dose-intensive chemotherapy or amrubicin plus cisplatin chemotherapy following induction chemoradiotherapy for limited-disease small cell lung cancer (JCOG1011). Lung Cancer, 108:232-237, 2017
3. Niho S, Ohe Y, Ohmatsu H, Umemura S, Matsumoto S, Yoh K, Goto K. Switch maintenance chemotherapy using S-1 with or without bevacizumab in patients with advanced non-small cell lung cancer: a phase II study. Lung Cancer, 108:66-71, 2017
4. Yoshida A, Kobayashi E, Kubo T, Kodaira M, Motoi T, Motoi N, Yonemori K, Ohe Y, Watanabe SI, Kawai A, Kohno T, Kishimoto H, Ichikawa H, Hiraoka N. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol, 30:797-809, 2017
5. Hida T, Nishio M, Nogami N, Ohe Y, Nokihara H, Sakai H, Satouchi M, Nakagawa K, Takenoyama M, Isobe H, Fujita S, Tanaka H, Minato K, Takahashi T, Maemondo M, Takeda K, Saka H, Goto K, Atagi S, Hirashima T, Sumiyoshi N, Tamura T. Efficacy and safety of nivolumab in Japanese patients with advanced or recurrent squamous non-small cell lung cancer. Cancer Sci, 108:1000-1006, 2017
6. Shimomura A, Kondo S, Kobayashi N, Iwasa S, Kitano S, Tamura K, Fujiwara Y, Yamamoto N. Do all patients in the phase I oncology trials need to be hospitalized? Domestic but outstanding issues for globalization of drug development in Japan. Int J Clin Oncol, 22:780-785, 2017
7. Tamura T, Kiura K, Seto T, Nakagawa K, Maemondo M, Inoue A, Hida T, Yoshioka H, Harada M, Ohe Y, Nogami N, Murakami H, Kuriki H, Shimada T, Tanaka T, Takeuchi K, Nishio M. Three-Year Follow-Up of an Alectinib Phase I/II Study in ALK-Positive Non-Small-Cell Lung Cancer: AF-001JP. J Clin Oncol, 35:1515-1521, 2017
8. Yoh K, Goto Y, Naito Y, Kishi K, Mori K, Hotta K, Hosomi Y, Yamada K, Tanai C, Tomizawa Y, Inoue A, Hasegawa Y, Nishio M, Ohashi Y, Kunitoh H. Impact of Maintenance Therapy for Patients with Non-small Cell Lung Cancer in a Real-world Setting. Anticancer Res, 37:1507-1513, 2017
9. Ohe Y, Zielinski C. ESMO Open welcomes the association with the Japanese Society of Medical Oncology (JSMO). ESMO open, 2:e000240, 2017
10. Konagai A, Yoshimura K, Hazama S, Yamamoto N, Aoki K, Ueno T, Fujioka M, Iijima H, Kato M, Uchida M, Wada T, Inoue M, Asao T, Fuse M, Wada S, Kuramasu A, Kamei R, Takeda S, Yamamoto S, Yoshino S, Oka M, Nagano H. Correlation Between NKG2DL Expression and Antitumor Effect of Protein-bound Polysaccharide-K in Tumor-bearing Mouse Models. Anticancer Res, 37:4093-4101, 2017
11. Inoki K, Sakamoto T, Takamaru H, Sekiguchi M, Yamada M, Nakajima T, Matsuda T, Taniguchi H, Sekine S, Kanemitsu Y, Ohe Y, Saito Y. Predictive relevance of lymphovascular invasion in T1 colorectal cancer before endoscopic treatment. Endoscopy international open, 5:E1278-E1283, 2017
12. Goto Y. Another disappointing result, but how good is it? Journal of thoracic disease, 9:1426-1428, 2017
13. Yamamoto N, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Tanabe Y, Horiike A, Yanagitani N, Kitazono S, Inatani M, Tanaka J, Nishio M. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs, 35:37-46, 2017
14. Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Tajima K, Tamura T. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol, 22:70-78, 2017
15. Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Tajima K, Tamura T. Erratum to: Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol, 22:79, 2017
16. Horinouchi H. KEYNOTE-010: flash of a supernova (immune checkpoint inhibitors) in second-line non-small cell lung cancer. Journal of thoracic disease, 9:4187-4190, 2017
17. Asao T, Fujiwara Y, Itahashi K, Kitahara S, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Takahashi K, Ohe Y. Sequential Use of Anaplastic Lymphoma Kinase Inhibitors in Japanese Patients With ALK-Rearranged Non-Small-Cell Lung Cancer: A Retrospective Analysis. Clin Lung Cancer, 18:e251-e258, 2017
18. Okuma HS, Horinouchi H, Kitahara S, Asao T, Sunami K, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Comparison of Amrubicin and Weekly Cisplatin/Etoposide/Irinotecan in Patients With Relapsed Small-cell Lung Cancer. Clin Lung Cancer, 18:234-240 e232, 2017
19. Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H, Tamura T. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs, 35:207-216, 2017
20. Fujiwara Y, Goto Y, Kanda S, Horinouchi H, Yamamoto N, Sakiyama N, Ando Makihara R, Ohe Y. Efficacy and safety of osimertinib in a Japanese compassionate use program. Jpn J Clin Oncol, 47:625-629, 2017
21. Overcoming resistance to third-generation epidermal growth factor receptor tyrosine kinase inhibitor in non-small cell lung cancer. Transl Cancer Res, 6:S1187-S1190, 2017
22. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 5:42-50, 2017
23. Kubo E, Yamamoto N, Nokihara H, Fujiwara Y, Horinouchi H, Kanda S, Goto Y, Ohe Y. Randomized phase II study of sequential carboplatin plus paclitaxel and gefitinib in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: Long-term follow-up results. Molecular and clinical oncology, 6:56-62, 2017
24. Sasada S, Ushirozawa N, Kobayashi N, Fujiwara Y, Tamura K, Yamamoto N. Surveillance of protocol deviations in Japanese oncology registration trials: a single institute experience. Invest New Drugs, 35:392-396, 2017
25. Nokihara H, Yamamoto N, Yamada Y, Honda K, Asahina H, Tamura Y, Hozak RR, Gao L, Suzukawa K, Enatsu S, Tamura T. A phase 1 study of ramucirumab in Japanese patients with advanced solid tumors. Jpn J Clin Oncol, 47:298-305, 2017
26. Wang F, Mishina S, Takai S, Le TK, Ochi K, Funato K, Matsuoka S, Ohe Y. Systemic Treatment Patterns With Advanced or Recurrent Non-small Cell Lung Cancer in Japan: A Retrospective Hospital Administrative Database Study. Clin Ther, 39:1146-1160, 2017
27. Saito M, Fujiwara Y, Asao T, Honda T, Shimada Y, Kanai Y, Tsuta K, Kono K, Watanabe S, Ohe Y, Kohno T. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis, 38:1084-1091, 2017
28. Goto Y, Tanai C, Yoh K, Hosomi Y, Sakai H, Kato T, Kaburagi T, Nishio M, Kim YH, Inoue A, Hasegawa Y, Isobe H, Tomizawa Y, Mori Y, Minato K, Yamada K, Ohashi Y, Kunitoh H. Continuing EGFR-TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation-positive non-small-cell lung cancer: an observational study. ESMO open, 2:e000214, 2017
29. Nakamichi S, Horinouchi H, Asao T, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ito Y, Watanabe SI, Ohe Y. Comparison of Radiotherapy and Chemoradiotherapy for Locoregional Recurrence of Non-small-cell Lung Cancer Developing After Surgery. Clin Lung Cancer, 18:e441-e448, 2017
30. Wu YL, Saijo N, Thongprasert S, Yang JC, Han B, Margono B, Chewaskulyong B, Sunpaweravong P, Ohe Y, Ichinose Y, Yang JJ, Mok TS, Young H, Haddad V, Rukazenkov Y, Fukuoka M. Efficacy according to blind independent central review: Post-hoc analyses from the phase III, randomized, multicenter, IPASS study of first-line gefitinib versus carboplatin/paclitaxel in Asian patients with EGFR mutation-positive advanced NSCLC. Lung Cancer, 104:119-125, 2017
31. Kamei R, Yoshimura K, Yoshino S, Inoue M, Asao T, Fuse M, Wada S, Kuramasu A, Furuya-Kondo T, Oga A, Iizuka N, Suzuki N, Maeda N, Watanabe Y, Matsukuma S, Iida M, Takeda S, Ueno T, Yamamoto N, Fukagawa T, Katai H, Sasaki H, Hazama S, Oka M, Nagano H. Expression levels of UL16 binding protein 1 and natural killer group 2 member D affect overall survival in patients with gastric cancer following gastrectomy. Oncol Lett, 15:747-754, 2018
32. Seki Y, Fujiwara Y, Kohno T, Yoshida K, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Kuwano K, Ohe Y. Circulating cell-free plasma tumour DNA shows a higher incidence of EGFR mutations in patients with extrathoracic disease progression. ESMO Open, 3:e000292, 2018
33. Okamoto I, Morita S, Tashiro N, Imamura F, Inoue A, Seto T, Yamamoto N, Ohe Y, Nakagawa K, Fukuoka M. Real world treatment and outcomes in EGFR mutation-positive non-small cell lung cancer: Long-term follow-up of a large patient cohort. Lung Cancer, 117:14-19, 2018
34. Horinouchi H, Kubota K, Miyanaga A, Nakamichi S, Seike M, Gemma A, Yamane Y, Kurimoto F, Sakai H, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Tamura T, Ohe Y. Oral rehydration solution (OS-1) as a substitute of intravenous hydration after cisplatin administration in patients with lung cancer: a prospective multicenter trial. ESMO Open, 3:e000288, 2018
35. Sato J, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Long-term survival without surgery in NSCLC patients with synchronous brain oligometastasis: systemic chemotherapy revisited. J Thorac Dis, 10:1696-1702, 2018
36. Ramalingam SS, Yang JC, Lee CK, Kurata T, Kim DW, John T, Nogami N, Ohe Y, Mann H, Rukazenkov Y, Ghiorghiu S, Stetson D, Markovets A, Barrett JC, Thress KS, Janne PA. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol, 36:841-849, 2018
37. Kato T, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tao L, Yu W, Khaznadar T, Tajima K, Shibata M, Seki A. Erlotinib Plus Bevacizumab Phase ll Study in Patients with Advanced Non-small-Cell Lung Cancer (JO25567): Updated Safety Results. Drug Saf, 41:229-237, 2018
38. Ohara S, Kanda S, Okuma H, Goto Y, Horinouchi H, Fujiwara Y, Nokihara H, Ito Y, Yamamoto N, Usui K, Homma S, Ohe Y. Effect of sequential chemoradiotherapy in patients with limited-disease small-cell lung cancer who were ineligible for concurrent therapy: a retrospective study at two institutions. Jpn J Clin Oncol, 48:82-88, 2018
39. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med, 378:113-125, 2018
40. Taira T, Yoh K, Nagase S, Kubota K, Ohmatsu H, Niho S, Onozawa M, Akimoto T, Ohe Y, Goto K. Long-term results of S-1 plus cisplatin with concurrent thoracic radiotherapy for locally advanced non-small-cell lung cancer. Cancer Chemother Pharmacol, 81:565-572, 2018
