Annual Report 2017
Department of Hepatobiliary and Pancreatic Oncology
Takuji Okusaka, Chigusa Morizane, Susumu Hijioka, Shunsuke Kondo, Yasunari Sakamoto, Akihiro Ohba, Yuta Maruki, Yoshikuni Nagashio
Introduction
The Department of Hepatobiliary and Pancreatic Oncology treats tumors originating from the liver, biliary system, or pancreas, which include hepatocellular carcinoma (HCC), biliary tract can-cer and pancreatic cancer. As part of the multi- disciplinary care given at the National Cancer Center Hospital (NCCH), we work closely with surgeons and radiologists who have special expertise in these areas. We also conduct research into the pathophysiology of hepatobiliary and pancreatic tumors and seek to develop new and more effective diagnostic methods and treatments.
Our team and what we do
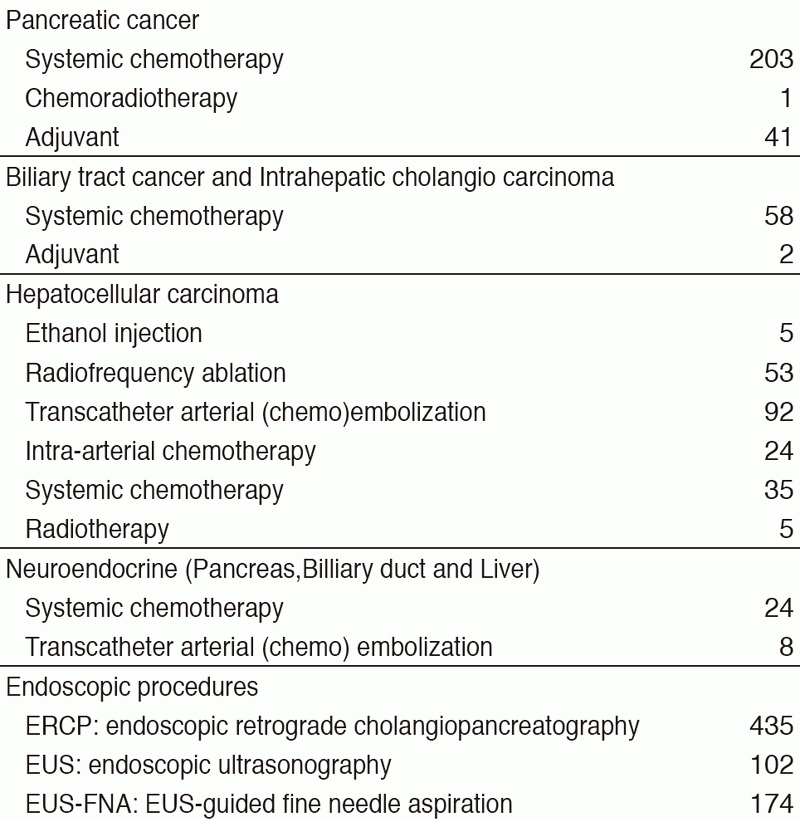
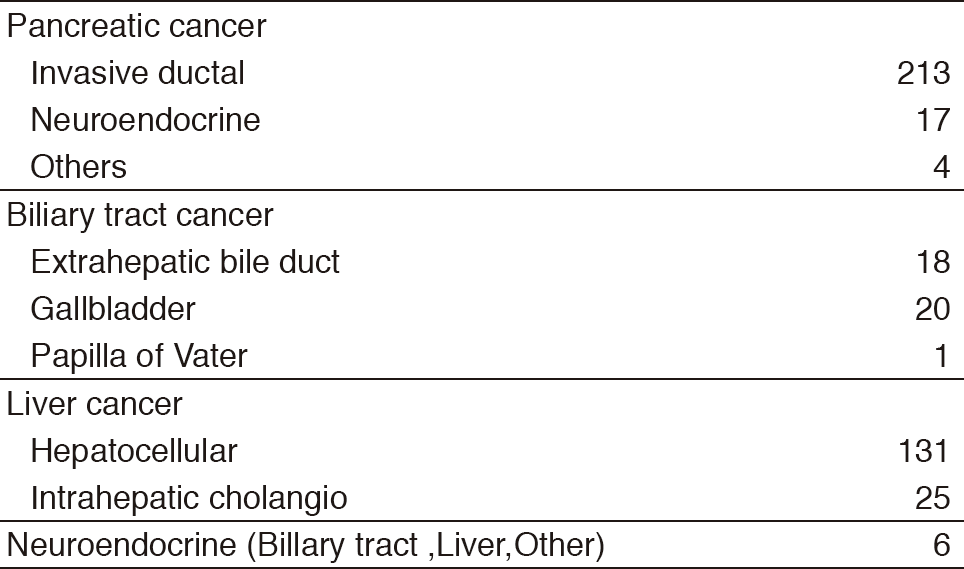
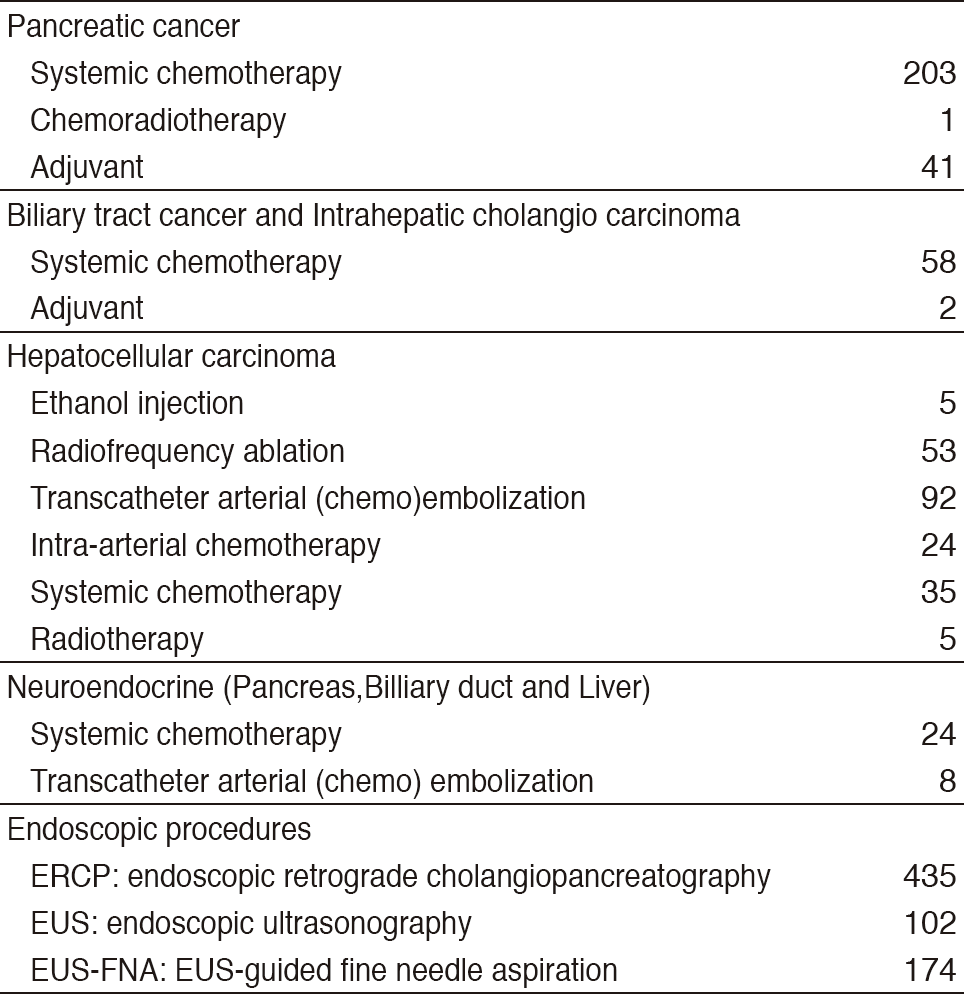
Our department consists of five staff oncologists and several residents. We have used percutaneous ablation therapy as a valuable alternative to surgery for most patients with three or fewer HCC nodules, all of which are smaller than 3 cm in diameter. We also perform transcatheter arterial chemoembolization (TACE), mainly in patients with multiple HCC nodules. Systemic or intra-arterial chemotherapeutic regimens are indicated in advanced HCC patients for whom locoregional intervention and surgery are unsuitable or unsuccessful. In patients with unresectable pancreatic cancer or biliary tract cancer, chemotherapy is performed in clinical practice or as a clinical trial to develop active treatment. We have actively introduced endoscopic procedures for imaging diagnosis (endoscopic ultrasonography: EUS, endoscopic retrograde cholangiopancreatography: ERCP), tumor biopsy (EUS-guided fine needle aspiration: EUS-FNA), and biliary drainage including EUS-guided hepaticogastrostomy (EUS-HGS) and EUS-guided choledochoduodenostomy (EUS-CDS) (Tables 1 & 2).
Table 1. Number of patients ( Jan/2017-Mar/2018)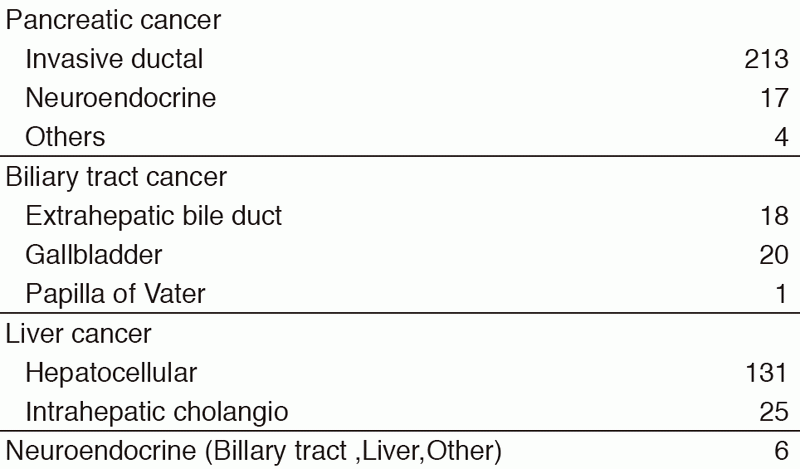
Research activities
We performed a randomized three-arm phase III study to evaluate the non-inferiority of S-1 monotherapy and superiority of gemcitabine plus S-1 (GS) to gemcitabine in patients with locally advanced and metastatic pancreatic cancer (GEST study). In the previous analysis, overall survival (OS) was estimated on the basis of 710 deaths (85.3%) among the 832 patients (median follow-up, 18.4 months). In the present study, we followed-up the survivors and updated the OS data to obtain more robust conclusions. The median follow-up period was 29.8 months, and 795 deaths occurred (95.6%). The median overall survival was 8.8 months for gemcitabine, 9.7 months for S-1 (hazard ratio [HR], 0.96; 97.5% confidence interval [CI], 0.79-1.17), and 9.9 months for GS (HR 0.91; 97.5% CI 0.75-1.11). In patients with performance status (PS) 0, the median overall survival was 9.8 months for gemcitabine, 10.9 months for S-1, and 10.5 months for GS. In patients with PS 1, the median overall survival was 6.2 months for gemcitabine, 6.3 months for S-1, and 9.6 months for GS (Okusaka et al., 2017).
We examined the clinicopathologic and molecular features of pancreatic neuroendocrine neoplasm grade-3 (PanNEN-G3) and assessed the responsiveness to chemotherapy and survival. Seventy patients analyzed included 21 well-differentiated neuroendocrine tumor with G3 (NET-G3) (30%) and 49 NECs-G3 (70%). NET-G3 showed lower Ki67-labeling index (LI; median 28.5%), no abnormal Rb expression (0%), and no mutated KRAS (0%), whereas NEC-G3 showed higher Ki67-LI (median 80.0%), Rb loss (54.5%), and KRAS mutations (48.7%). Chemotherapy response rate (RR), platinum-based chemotherapy RR, and prognosis differed significantly between NET-G3 and NEC-G3. Chemotherapeutic outcomes were worse in NET-G3 (P < 0.001). When we stratified PanNEN-G3 with Rb and KRAS, PanNENs-G3 with Rb loss and those with mutated KRAS showed significantly higher RRs to platinum-based chemotherapy than those without (Rb loss, 80% vs. normal Rb, 24%, P = 0.006; mutated KRAS, 77% versus wild type, 23%, P = 0.023). Rb was a predictive marker of response to platinum-based chemotherapy even in NEC-G3 (P = 0.035) (Hijioka et al., 2017).
Clinical trials
Twenty six clinical trials are ongoing, including fourteen phase I or I/II trials, eight phase II or II/III trials, and four phase III trials such as adjuvant chemotherapy after resection versus resection alone for patients with resectable tumors, and chemotherapy with a new regimen versus standard therapy for patients with advanced tumors. Our studies are supported by the National Cancer Center Research and Development Fund (Grant No. 29-A-3), and Project for Development of Innovative Research on Cancer Therapeutics (P-DIRECT: 17ck0106350h0001, 17ck0106355h0001, 17ck0106354h0001) from the Japan Agency for Medical Research and Development (AMED).
Education
Our staff members are working closely with residents to support their skill development and knowledge expansion in both clinical and research fields. We are conducting conferences daily for clinical practice and weekly for research development. The residents in our department have published two papers as first authors in peer-reviewed journals in 2017, and are performing nineteen ongoing studies as leading researchers, with assistance from staff members.
Future prospects
Our department keeps providing the best and latest diagnosis, treatment and supportive care, and developing more effective methods and techniques for all patients with hepatobiliary and pancreatic cancers in this country and all over the world. Among them, conducting clinical trials with novel promising agents for these diseases is considered one of the most important tasks, and the establishment of cutting-edge endoscopic procedures in this field is the most significant mission for us.
List of papers published in January 2017 - March 2018
Journal
1.Matsubayashi H, Takaori K, Morizane C, Maguchi H, Mizuma M, Takahashi H, Wada K, Hosoi H, Yachida S, Suzuki M, Usui R, Furukawa T, Furuse J, Sato T, Ueno M, Kiyozumi Y, Hijioka S, Mizuno N, Terashima T, Mizumoto M, Kodama Y, Torishima M, Kawaguchi T, Ashida R, Kitano M, Hanada K, Furukawa M, Kawabe K, Majima Y, Shimosegawa T. Familial pancreatic cancer: Concept, management and issues. World journal of gastroenterology, 23:935-948, 2017
2.Kobayashi S, Ueno M, Sugimori K, Morizane C, Kojima Y, Irie K, Goda Y, Morimoto M, Ohkawa S. Phase II study of fixed dose-rate gemcitabine plus S-1 as a second-line treatment for advanced biliary tract cancer. Cancer Chemother Pharmacol, 80:1189-1196, 2017
3.Ueno M, Li CP, Ikeda M, Ishii H, Mizuno N, Yamaguchi T, Ioka T, Oh DY, Ichikawa W, Okusaka T, Matsuyama Y, Arai D, Chen LT, Park YS, Furuse J. A randomized phase II study of gemcitabine plus Z-360, a CCK2 receptor-selective antagonist, in patients with metastatic pancreatic cancer as compared with gemcitabine plus placebo. Cancer Chemother Pharmacol, 80:307-315, 2017
4.Kudo M, Hatano E, Ohkawa S, Fujii H, Masumoto A, Furuse J, Wada Y, Ishii H, Obi S, Kaneko S, Kawazoe S, Yokosuka O, Ikeda M, Ukai K, Morita S, Tsuji A, Kudo T, Shimada M, Osaki Y, Tateishi R, Sugiyama G, Abada PB, Yang L, Okusaka T, Zhu AX. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma: Japanese subgroup analysis of the REACH trial. J Gastroenterol, 52:494-503, 2017
5.Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, Okita K, Kumada H. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol, 52:512-519, 2017
6.Kondo S, Hosoi H, Itahashi K, Hashimoto J. Quality evaluation of investigator-initiated trials using post-approval cancer drugs in Japan. Cancer Sci, 108:995-999, 2017
7.Okuyama H, Ikeda M, Takahashi H, Ohno I, Hashimoto Y, Mitsunaga S, Sakamoto Y, Kondo S, Morizane C, Ueno H, Kobayashi T, Arai Y, Okusaka T. Transarterial (Chemo)Embolization for Liver Metastases in Patients with Neuroendocrine Tumors. Oncology, 92:353-359, 2017
8.Ikeda M, Okusaka T, Sato Y, Furuse J, Mitsunaga S, Ueno H, Morizane C, Inaba Y, Kobayashi T, Arai Y. A Phase I/II trial of continuous hepatic intra-arterial infusion of 5-fluorouracil, mitoxantrone and cisplatin for advanced hepatocellular carcinoma. Jpn J Clin Oncol, 47:512-519, 2017
9.McNamara MG, Bridgewater J, Lopes A, Wasan H, Malka D, Jensen LH, Okusaka T, Knox JJ, Wagner D, Cunningham D, Shannon J, Goldstein D, Moehler M, Bekaii-Saab T, Valle JW. Systemic therapy in younger and elderly patients with advanced biliary cancer: sub-analysis of ABC-02 and twelve other prospective trials. BMC Cancer, 17:262, 2017
10.Hagiwara Y, Ohashi Y, Okusaka T, Ueno H, Ioka T, Boku N, Egawa S, Hatori T, Furuse J, Mizumoto K, Ohkawa S, Yamaguchi T, Yamao K, Funakoshi A, Cheng AL, Kihara K, Sato A, Tanaka M. Health-related quality of life in a randomised phase III study of gemcitabine plus S-1, S-1 alone and gemcitabine alone for locally advanced or metastatic pancreatic cancer: GEST study. ESMO Open, 2:e000151, 2017
11.Shimada Y, Kohno T, Ueno H, Ino Y, Hayashi H, Nakaoku T, Sakamoto Y, Kondo S, Morizane C, Shimada K, Okusaka T, Hiraoka N. An Oncogenic ALK Fusion and an RRAS Mutation in KRAS Mutation-Negative Pancreatic Ductal Adenocarcinoma. Oncologist, 22:158-164, 2017
12.Hayashi H, Kohno T, Ueno H, Hiraoka N, Kondo S, Saito M, Shimada Y, Ichikawa H, Kato M, Shibata T, Morizane C, Sakamoto Y, Shimada K, Komatsu Y, Sakamoto N, Okusaka T. Utility of Assessing the Number of Mutated KRAS, CDKN2A, TP53, and SMAD4 Genes Using a Targeted Deep Sequencing Assay as a Prognostic Biomarker for Pancreatic Cancer. Pancreas, 46:335-340, 2017
13.Yamaguchi K, Okusaka T, Shimizu K, Furuse J, Ito Y, Hanada K, Shimosegawa T, Okazaki K. Clinical Practice Guidelines for Pancreatic Cancer 2016 From the Japan Pancreas Society: A Synopsis. Pancreas, 46:595-604, 2017
14.Kimura K, Ikoma A, Shibakawa M, Shimoda S, Harada K, Saio M, Imamura J, Osawa Y, Kimura M, Nishikawa K, Okusaka T, Morita S, Inoue K, Kanto T, Todaka K, Nakanishi Y, Kohara M, Mizokami M. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/beta-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine, 23:79-87, 2017
15.Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, Kobayashi N, Ikeda M, Ito T, Nakamori S, Ishii H, Kodama Y, Morizane C, Okusaka T, Yanagimoto H, Notohara K, Taguchi H, Kitano M, Yane K, Maguchi H, Tsuchiya Y, Komoto I, Tanaka H, Tsuji A, Hashigo S, Kawaguchi Y, Mine T, Kanno A, Murohisa G, Miyabe K, Takagi T, Matayoshi N, Yoshida T, Hara K, Imamura M, Furuse J, Yatabe Y, Mizuno N. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clinical cancer research, 23:4625-4632, 2017
16.Hamada C, Okusaka T, Ikari T, Isayama H, Furuse J, Ishii H, Nakai Y, Imai S, Okamura S. Efficacy and safety of gemcitabine plus S-1 in pancreatic cancer: a pooled analysis of individual patient data. Br J Cancer, 116:1544-1550, 2017
17.Hijioka S, Hosoda W, Morizane C, Mizuno N, Hara K, Okusaka T. The Diagnosis and Treatment of Pancreatic NEN-G3-A Focus on Clinicopathological Difference of NET-G3 and NEC G3. JOP; J Pancreas (Online), S:216-220, 2017
18.Kudo M, Moriguchi M, Numata K, Hidaka H, Tanaka H, Ikeda M, Kawazoe S, Ohkawa S, Sato Y, Kaneko S, Furuse J, Takeuchi M, Fang X, Date Y, Okusaka T. S-1 versus placebo in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE): a randomised, double-blind, multicentre, phase 3 trial. The lancet. Gastroenterology & hepatology, 2:407-417, 2017
19.Ikeda M, Shimizu S, Sato T, Morimoto M, Kojima Y, Inaba Y, Hagihara A, Kudo M, Nakamori S, Kaneko S, Sugimoto R, Tahara T, Ohmura T, Yasui K, Sato K, Ishii H, Furuse J, Okusaka T. Reply to the Letter to the editor 'Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus Sorafenib for advanced hepatocellular carcinoma: randomized phase II trial' by Fornaro et al. Ann Oncol, 28:903-904, 2017
20.Arnold D, Fuchs CS, Tabernero J, Ohtsu A, Zhu AX, Garon EB, Mackey JR, Paz-Ares L, Baron AD, Okusaka T, Yoshino T, Yoon HH, Das M, Ferry D, Zhang Y, Lin Y, Binder P, Sashegyi A, Chau I. Meta-analysis of individual patient safety data from six randomized, placebo-controlled trials with the antiangiogenic VEGFR2-binding monoclonal antibody ramucirumab. Ann Oncol, 28:2932-2942, 2017
21.Furuse J, Gemma A, Ichikawa W, Okusaka T, Seki A, Ishii T. Postmarketing surveillance study of erlotinib plus gemcitabine for pancreatic cancer in Japan: POLARIS final analysis. Jpn J Clin Oncol, 47:832-839, 2017
22.Tamai T, Hayato S, Hojo S, Suzuki T, Okusaka T, Ikeda K, Kumada H. Dose Finding of Lenvatinib in Subjects With Advanced Hepatocellular Carcinoma Based on Population Pharmacokinetic and Exposure-Response Analyses. J Clin Pharmacol, 57:1138-1147, 2017
23.Okusaka T, Miyakawa H, Fujii H, Nakamori S, Satoh T, Hamamoto Y, Ito T, Maguchi H, Matsumoto S, Ueno H, Ioka T, Boku N, Egawa S, Hatori T, Furuse J, Mizumoto K, Ohkawa S, Yamaguchi T, Yamao K, Funakoshi A, Chen JS, Cheng AL, Sato A, Ohashi Y, Tanaka M. Updated results from GEST study: a randomized, three-arm phase III study for advanced pancreatic cancer. J Cancer Res Clin Oncol, 143:1053-1059, 2017
24.Hirata Y, Kobayashi T, Nishiumi S, Yamanaka K, Nakagawa T, Fujigaki S, Iemoto T, Kobayashi M, Okusaka T, Nakamori S, Shimahara M, Ueno T, Tsuchida A, Sata N, Ioka T, Yasunami Y, Kosuge T, Kaneda T, Kato T, Yagihara K, Fujita S, Yamada T, Honda K, Azuma T, Yoshida M. Identification of highly sensitive biomarkers that can aid the early detection of pancreatic cancer using GC/MS/MS-based targeted metabolomics. Clin Chim Acta, 468:98-104, 2017
25.Chau I, Peck-Radosavljevic M, Borg C, Malfertheiner P, Seitz JF, Park JO, Ryoo BY, Yen CJ, Kudo M, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Okusaka T, Bowman L, Cui ZL, Girvan AC, Abada PB, Yang L, Zhu AX. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: Patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer, 81:17-25, 2017
26.Iwata T, Ueno H, Itami J, Ito Y, Inaba K, Morizane C, Kondo S, Sakamoto Y, Shiba S, Sasaki M, Koga F, Okusaka T. Efficacy of radiotherapy for primary tumor in patients with unresectable pancreatic neuroendocrine tumors. Jpn J Clin Oncol, 47:826-831, 2017
27.Ter Veer E, van Rijssen LB, Besselink MG, Mali RMA, Berlin JD, Boeck S, Bonnetain F, Chau I, Conroy T, Van Cutsem E, Deplanque G, Friess H, Glimelius B, Goldstein D, Herrmann R, Labianca R, Van Laethem JL, Macarulla T, van der Meer JHM, Neoptolemos JP, Okusaka T, O'Reilly EM, Pelzer U, Philip PA, van der Poel MJ, Reni M, Scheithauer W, Siveke JT, Verslype C, Busch OR, Wilmink JW, van Oijen MGH, van Laarhoven HWM. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM-PACT) for systemic treatment of unresectable disease. Lancet Oncol, 19:e151-e160, 2018
28.Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, Aikata H, Nagano H, Hatano E, Sasaki Y, Hino K, Kumada T, Yamamoto K, Imai Y, Iwadou S, Ogawa C, Okusaka T, Kanai F, Akazawa K, Yoshimura KI, Johnson P, Arai Y. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol, 3:424-432, 2018
29.Morita S, Arai Y, Sugawara S, Sone M, Sakamoto Y, Okusaka T, Yoshinaga S, Saito Y, Terai S. Antireflux Metal Stent for Initial Treatment of Malignant Distal Biliary Obstruction. Gastroenterol Res Pract, 2018:3805173, 2018
30.Kondo S, Sasaki M, Hosoi H, Sakamoto Y, Morizane C, Ueno H, Okusaka T. Incidence and risk factors for venous thromboembolism in patients with pretreated advanced pancreatic carcinoma. Oncotarget, 9:16883-16890, 2018
31.Ikeda M, Kudo M, Aikata H, Nagamatsu H, Ishii H, Yokosuka O, Torimura T, Morimoto M, Ikeda K, Kumada H, Sato T, Kawai I, Yamashita T, Horio H, Okusaka T. Transarterial chemoembolization with miriplatin vs. epirubicin for unresectable hepatocellular carcinoma: a phase III randomized trial. J Gastroenterol, 53:281-290, 2018
32.Ishimoto U, Kondo S, Ohba A, Sasaki M, Sakamoto Y, Morizane C, Ueno H, Okusaka T. Prognostic Factors for Survival in Patients with Advanced Intrahepatic Cholangiocarcinoma Treated with Gemcitabine plus Cisplatin as First-Line Treatment. Oncology, 94:72-78, 2018
33.Ikeda M, Ioka T, Fukutomi A, Morizane C, Kasuga A, Takahashi H, Todaka A, Okusaka T, Creasy CL, Gorman S, Felitsky DJ, Kobayashi M, Zhang F, Furuse J. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci, 109:215-224, 2018
34.Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J. Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol, 48:103-114, 2018