Annual Report 2017
Department of Experimental Therapeutics
Noboru Yamamoto, Toshio Shimizu, Yutaka Fujiwara, Kan Yonemori, Kiyoshi Yoshimura, Shigehisa Kitano, Shunsuke Kondo, Satoru Iwasa, Akihiko Shimomura, Takafumi Koyama, Takahiro Ebata
Introduction
In April 2015, the affiliation of the Department of Experimental Therapeutics was changed from the National Cencer Center (NCC) - the Exploratory Oncology Research & Clinical Trial Center (EPOC) to the NCC-Hospital. The goal of our department is to perform initial clinical evaluation of promising new anti-cancer compounds emerging from the laboratory in phase I trials. The staff of this department consists of specialists from various oncology fields (i.e. thoracic oncology, breast and medical oncology, gastro-intestinal oncology, hepato-biliary and pancreatic oncology, and immuno-oncology).
Our team and what we do
This department plays a key role of the new anti-cancer drug development in Japan as well as in Asia. The top priority is to conduct the FIH trials, and is to also perform the phase I trials for solid tumors (i.e. all comers). Recently, we have been joining the global phase I trials to accelerate the new drug development in Japan. Web- or tel -conferences are held with the EU and US sites, and we discuss patient enrollment as well as further developmental strategy. Routine web-
conferences are also held between the NCC-
Hospital (NCCH, Tokyo) and the NCC-Hospital East (NCCHE, Chiba) every Friday morning, and we share information about adverse events, patient enrollment, and refer candidates to each other to accelerate enrollment. Nowadays, the most of the phase I trials (i.e. first in Japanese phase I trial, first in human trial) in Japan are conducted at the NCCH and the NCCHE in close collaboration.
Research activities
The elucidation of the proof of concept is essential in the new anti-cancer drug development especially in the early phase, so we conduct several translational researches (TR) in collaboration with the adjoining NCC - Research Institute. Comprehensive genomic analyses, named TOP-GEAR-study (UMIN000011141), are ongoing to facilitate patient enrollment for new molecular targeted drugs under investigation. Also, we are conducting the TR with the pharmaceutical industry to discover new targets for anti-immune therapy using human tissue (tumor and normal tissue) samples.
Clinical trials
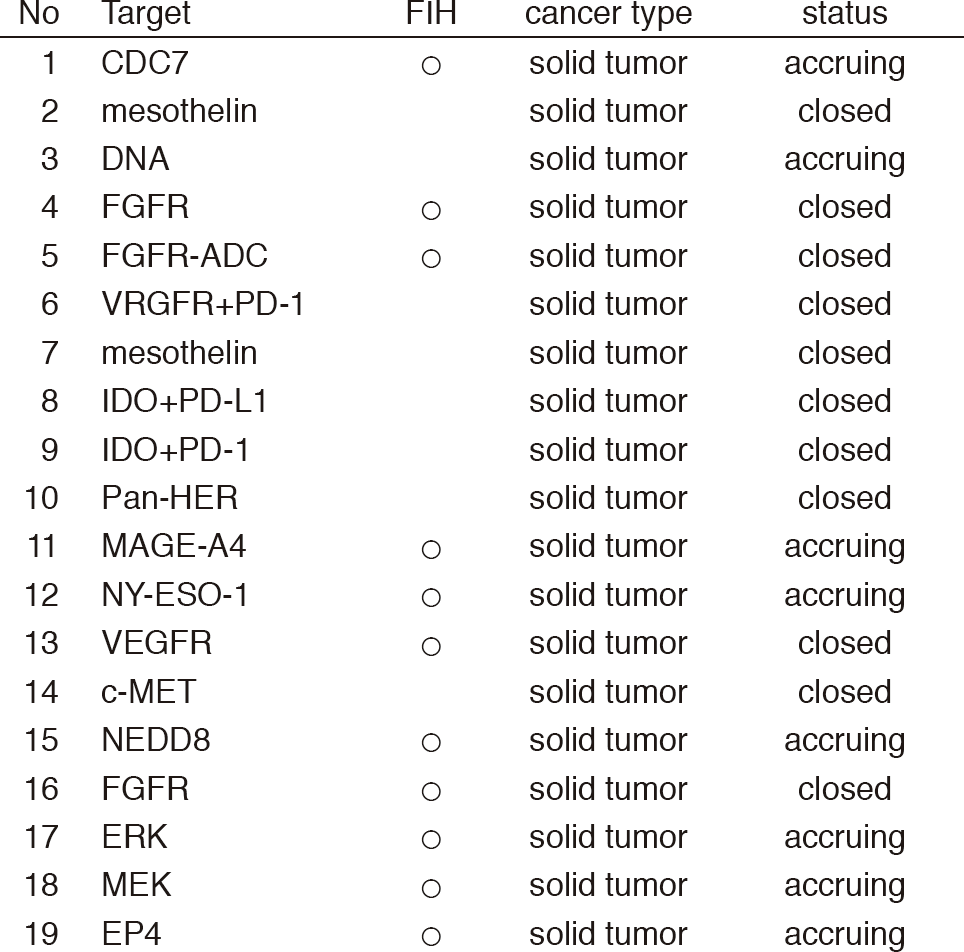
From Jan/2017 to Mar/2018, 39 phase I trials including 17 FIH trials were conducted (Table 1).
Table 1: Phase I trials conducted from Jan/2017 to Mar/2018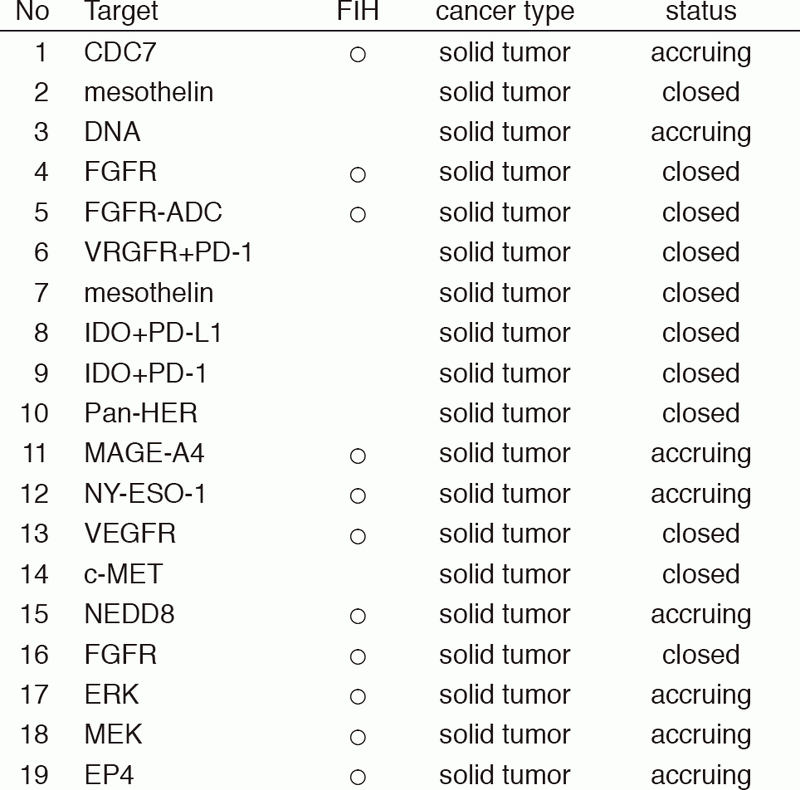
Education
In 2017, the Second NCCH Workshop on Methods in Oncology Phase I Trials and Translational Research was held on July 23 at the NCC-Tsukiji Campus.
List of papers published in January 2017 - March 2018
Journal
1. Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Asakura K, Nakagawa K, Sakurai H, Watanabe SI, Tsuta K, Ohe Y. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer, 108:115-120, 2017
2. Sekine I, Harada H, Yamamoto N, Wakabayashi M, Murakami H, Goto K, Nogami N, Seto T, Oshita F, Okamoto H, Tanaka H, Tamura T, Ishikura S, Ohe Y. Randomized phase II trial of weekly dose-intensive chemotherapy or amrubicin plus cisplatin chemotherapy following induction chemoradiotherapy for limited-disease small cell lung cancer (JCOG1011). Lung Cancer, 108:232-237, 2017
3. Yoshida A, Kobayashi E, Kubo T, Kodaira M, Motoi T, Motoi N, Yonemori K, Ohe Y, Watanabe SI, Kawai A, Kohno T, Kishimoto H, Ichikawa H, Hiraoka N. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol, 30:797-809, 2017
4. Saka H, Kitagawa C, Kogure Y, Takahashi Y, Fujikawa K, Sagawa T, Iwasa S, Takahashi N, Fukao T, Tchinou C, Landers D, Yamada Y. Safety, tolerability and pharmacokinetics of the fibroblast growth factor receptor inhibitor AZD4547 in Japanese patients with advanced solid tumours: a Phase I study. Invest New Drugs, 35:451-462, 2017
5. Taniguchi H, Iwasa S, Yamazaki K, Yoshino T, Kiryu C, Naka Y, Liew EL, Sakata Y. Phase 1 study of OCV-C02, a peptide vaccine consisting of two peptide epitopes for refractory metastatic colorectal cancer. Cancer Sci, 108:1013-1021, 2017
6. Kubo E, Yamamoto N, Nokihara H, Fujiwara Y, Horinouchi H, Kanda S, Goto Y, Ohe Y. Randomized phase II study of sequential carboplatin plus paclitaxel and gefitinib in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: Long-term follow-up results. Mol Clin Oncol, 6:56-62, 2017
7. Ohue M, Iwasa S, Kanemitsu Y, Hamaguchi T, Shiozawa M, Ito M, Yasui M, Katayama H, Mizusawa J, Shimada Y. A Phase II/III randomized controlled trial comparing perioperative versus postoperative chemotherapy with mFOLFOX6 for lower rectal cancer with suspected lateral pelvic node metastasis: Japan Clinical Oncology Group Study JCOG1310 (PRECIOUS study). Jpn J Clin Oncol, 47:84-87, 2017
8. Nishikawa T, Matsumoto K, Tamura K, Yoshida H, Imai Y, Miyasaka A, Onoe T, Yamaguchi S, Shimizu C, Yonemori K, Shimoi T, Yunokawa M, Xiong H, Nuthalapati S, Hashiba H, Kiriyama T, Leahy T, Komarnitsky P, Fujiwara K. Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors. Cancer Sci, 108:1834-1842, 2017
9. Sakaida E, Ebata T, Iwasawa S, Kurimoto R, Yonemori S, Ota S, Nakatani Y, Sekine I, Takiguchi Y. Potential Activity of Amrubicin as a Salvage Therapy for Merkel Cell Carcinoma. Intern Med, 56:567-570, 2017
10. Shimomura A, Kondo S, Kobayashi N, Iwasa S, Kitano S, Tamura K, Fujiwara Y, Yamamoto N. Do all patients in the phase I oncology trials need to be hospitalized? Domestic but outstanding issues for globalization of drug development in Japan. Int J Clin Oncol, 22:780-785, 2017
11. Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci, 108:1109-1118, 2017
12. Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, Kitagawa Y. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol, 18:631-639, 2017
13. Takamochi K, Takahashi F, Suehara Y, Sato E, Kohsaka S, Hayashi T, Kitano S, Uneno T, Kojima S, Takeuchi K, Mano H, Suzuki K. DNA mismatch repair deficiency in surgically resected lung adenocarcinoma: Microsatellite instability analysis using the Promega panel. Lung Cancer, 110:26-31, 2017
14. Okuyama H, Ikeda M, Takahashi H, Ohno I, Hashimoto Y, Mitsunaga S, Sakamoto Y, Kondo S, Morizane C, Ueno H, Kobayashi T, Arai Y, Okusaka T. Transarterial (Chemo)Embolization for Liver Metastases in Patients with Neuroendocrine Tumors. Oncology, 92:353-359, 2017
15. Makise N, Yoshida A, Komiyama M, Nakatani F, Yonemori K, Kawai A, Fukayama M, Hiraoka N. Dedifferentiated Liposarcoma With Epithelioid/Epithelial Features. Am J Surg Pathol, 41:1523-1531, 2017
16. Watanabe S, Takashima A, Taniguchi H, Tanaka Y, Nakamura S, Okita N, Honma Y, Iwasa S, Kato K, Hamaguchi T, Boku N. Esophageal Metastasis from Rectal Cancer Successfully Treated with Fluorouracil-Based Chemotherapy with Bevacizumab: A Case Report and Review of the Literature. Case Rep Oncol, 10:407-415, 2017
17. Shoji H, Tada K, Kitano S, Nishimura T, Shimada Y, Nagashima K, Aoki K, Hiraoka N, Honma Y, Iwasa S, Takashima A, Kato K, Boku N, Honda K, Yamada T, Heike Y, Hamaguchi T. The peripheral immune status of granulocytic myeloid-derived suppressor cells correlates the survival in advanced gastric cancer patients receiving cisplatin-based chemotherapy. Oncotarget, 8:95083-95094, 2017
18. Yamamoto N, Fujiwara Y, Tamura K, Kondo S, Iwasa S, Tanabe Y, Horiike A, Yanagitani N, Kitazono S, Inatani M, Tanaka J, Nishio M. Phase Ia/Ib study of the pan-class I PI3K inhibitor pictilisib (GDC-0941) administered as a single agent in Japanese patients with solid tumors and in combination in Japanese patients with non-squamous non-small cell lung cancer. Invest New Drugs, 35:37-46, 2017
19. Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Tajima K, Tamura T. Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol, 22:70-78, 2017
20. Yamamoto N, Goto K, Nishio M, Chikamori K, Hida T, Maemondo M, Katakami N, Kozuki T, Yoshioka H, Seto T, Tajima K, Tamura T. Erratum to: Final overall survival in JO22903, a phase II, open-label study of first-line erlotinib for Japanese patients with EGFR mutation-positive non-small-cell lung cancer. Int J Clin Oncol, 22:79, 2017
21. Nishikawa K, Takahashi T, Takaishi H, Miki A, Noshiro H, Yoshikawa T, Nishida Y, Iwasa S, Miwa H, Masuishi T, Boku N, Yamada Y, Kodera Y, Yoshida K, Morita S, Sakamoto J, Saji S, Kitagawa Y. Phase II study of the effectiveness and safety of trastuzumab and paclitaxel for taxane- and trastuzumab-naive patients with HER2-positive, previously treated, advanced, or recurrent gastric cancer (JFMC45-1102). Int J Cancer, 140:188-196, 2017
22. Kurimoto R, Ebata T, Iwasawa S, Ishiwata T, Tada Y, Tatsumi K, Takiguchi Y. Pirfenidone may revert the epithelial-to-mesenchymal transition in human lung adenocarcinoma. Oncol Lett, 14:944-950, 2017
23. Matsui H, Hazama S, Sakamoto K, Shindo Y, Kanekiyo S, Nakashima M, Matsukuma S, Tokuhisa Y, Iida M, Suzuki N, Yoshimura K, Takeda S, Ueno T, Yoshino S, Oka M, Nagano H. Postoperative Adjuvant Therapy for Resectable Pancreatic Cancer With Gemcitabine and Adoptive Immunotherapy. Pancreas, 46:994-1002, 2017
24. Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, Kato K, Hamaguchi T, Yamada Y, Shimada Y, Boku N. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer, 20:655-662, 2017
25. Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, Shimomura A, Yonemori K, Shimizu C, Hamada A, Kanayama Y, Watanabe Y, Fujiwara Y, Tamura K. Visualization of HER2-specific breast cancer intratumoral heterogeneity using (64)Cu-DOTA-trastuzumab PET. Eur J Nucl Med Mol Imaging, 44:2146-2147, 2017
26. Shimada Y, Kohno T, Ueno H, Ino Y, Hayashi H, Nakaoku T, Sakamoto Y, Kondo S, Morizane C, Shimada K, Okusaka T, Hiraoka N. An Oncogenic ALK Fusion and an RRAS Mutation in KRAS Mutation-Negative Pancreatic Ductal Adenocarcinoma. Oncologist, 22:158-164, 2017
27. Terazawa T, Nishitani H, Kato K, Hashimoto H, Akiyoshi K, Ito Y, Nakamoto A, Iwasa S, Nakajima TE, Hamaguchi T, Yamada Y, Shimada Y. Phase II study of cetuximab with irinotecan for KRAS wild-type colorectal cancer in Japanese patients. Asia Pac J Clin Oncol, 13:e132-e137, 2017
28. Asao T, Fujiwara Y, Itahashi K, Kitahara S, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Takahashi K, Ohe Y. Sequential Use of Anaplastic Lymphoma Kinase Inhibitors in Japanese Patients With ALK-Rearranged Non-Small-Cell Lung Cancer: A Retrospective Analysis. Clin Lung Cancer, 18:e251-e258, 2017
29. Okuma HS, Horinouchi H, Kitahara S, Asao T, Sunami K, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Comparison of Amrubicin and Weekly Cisplatin/Etoposide/Irinotecan in Patients With Relapsed Small-cell Lung Cancer. Clin Lung Cancer, 18:234-240 e2, 2017
30. Yamamoto N, Nokihara H, Yamada Y, Shibata T, Tamura Y, Seki Y, Honda K, Tanabe Y, Wakui H, Tamura T. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs, 35:207-216, 2017
31. Tanabe Y, Shimizu C, Hamada A, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yamamoto H, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y. Paclitaxel-induced sensory peripheral neuropathy is associated with an ABCB1 single nucleotide polymorphism and older age in Japanese. Cancer Chemother Pharmacol, 79:1179-1186, 2017
32. Tanabe Y, Tsuda H, Yoshida M, Yunokawa M, Yonemori K, Shimizu C, Yamamoto S, Kinoshita T, Fujiwara Y, Tamura K. Pathological features of triple-negative breast cancers that showed progressive disease during neoadjuvant chemotherapy. Cancer Sci, 108:1520-1529, 2017
33. Yoh K, Seto T, Satouchi M, Nishio M, Yamamoto N, Murakami H, Nogami N, Matsumoto S, Kohno T, Tsuta K, Tsuchihara K, Ishii G, Nomura S, Sato A, Ohtsu A, Ohe Y, Goto K. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med, 5:42-50, 2017
34. Yoshida A, Arai Y, Kobayashi E, Yonemori K, Ogura K, Hama N, Mukai W, Motoi T, Kawai A, Shibata T, Hiraoka N. CIC break-apart fluorescence in-situ hybridization misses a subset of CIC-DUX4 sarcomas: a clinicopathological and molecular study. Histopathology, 71:461-469, 2017
35. Shimizu T, Yonesaka K, Hayashi H, Iwasa T, Haratani K, Yamada H, Ohwada S, Kamiyama E, Nakagawa K. Phase 1 study of new formulation of patritumab (U3-1287) Process 2, a fully human anti-HER3 monoclonal antibody in combination with erlotinib in Japanese patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol, 79:489-495, 2017
36. Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, Shimomura A, Yunokawa M, Yonemori K, Shimizu C, Hamada A, Kanayama Y, Watanabe Y, Fujiwara Y, Tamura K. 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann Oncol, 28:2028-2029, 2017
37. Sasada S, Ushirozawa N, Kobayashi N, Fujiwara Y, Tamura K, Yamamoto N. Surveillance of protocol deviations in Japanese oncology registration trials: a single institute experience. Invest New Drugs, 35:392-396, 2017
38. Nokihara H, Yamamoto N, Yamada Y, Honda K, Asahina H, Tamura Y, Hozak RR, Gao L, Suzukawa K, Enatsu S, Tamura T. A phase 1 study of ramucirumab in Japanese patients with advanced solid tumors. Jpn J Clin Oncol, 47:298-305, 2017
39. Yunokawa M, Yoshida H, Watanabe R, Noguchi E, Shimomura A, Shimoi T, Yonemori K, Shimizu C, Fujiwara Y, Tamura K. Allred score is a promising predictor of prognosis and medroxyprogesterone acetate efficacy in patients with endometrial cancer. Cancer Chemother Pharmacol, 80:127-134, 2017
40. Saito M, Fujiwara Y, Asao T, Honda T, Shimada Y, Kanai Y, Tsuta K, Kono K, Watanabe S, Ohe Y, Kohno T. The genomic and epigenomic landscape in thymic carcinoma. Carcinogenesis, 38:1084-1091, 2017
41. Nakamichi S, Horinouchi H, Asao T, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ito Y, Watanabe SI, Ohe Y. Comparison of Radiotherapy and Chemoradiotherapy for Locoregional Recurrence of Non-small-cell Lung Cancer Developing After Surgery. Clin Lung Cancer, 18:e441-e448, 2017
42. Yanai T, Iwasa S, Hashimoto H, Ohyanagi F, Takiguchi T, Takeda K, Nakao M, Sakai H, Nakayama T, Minato K, Arai T, Suzuki K, Shimada Y, Nagashima K, Terakado H, Yamamoto N. A double-blind randomized phase II dose-finding study of olanzapine 10 mg or 5 mg for the prophylaxis of emesis induced by highly emetogenic cisplatin-based chemotherapy. Int J Clin Oncol, 23:382-388, 2018
43. Kamei R, Yoshimura K, Yoshino S, Inoue M, Asao T, Fuse M, Wada S, Kuramasu A, Furuya-Kondo T, Oga A, Iizuka N, Suzuki N, Maeda N, Watanabe Y, Matsukuma S, Iida M, Takeda S, Ueno T, Yamamoto N, Fukagawa T, Katai H, Sasaki H, Hazama S, Oka M, Nagano H. Expression levels of UL16 binding protein 1 and natural killer group 2 member D affect overall survival in patients with gastric cancer following gastrectomy. Oncol Lett, 15:747-754, 2018
44. Yamazaki N, Tsutsumida A, Takahashi A, Namikawa K, Yoshikawa S, Fujiwara Y, Kondo S, Mukaiyama A, Zhang F, Kiyohara Y. Phase 1/2 study assessing the safety and efficacy of dabrafenib and trametinib combination therapy in Japanese patients with BRAF V600 mutation-positive advanced cutaneous melanoma. J Dermatol, 45:397-407, 2018
45. Kodaira M, Yonemori K, Shimoi T, Yoshida A, Yoshida M, Kitano A, Shimomura A, Yunokawa M, Shimizu C, Takiguchi Y, Fujiwara Y, Tamura K. Prognostic impact of presumed breast or ovarian cancer among patients with unfavorable-subset cancer of unknown primary site. BMC Cancer, 18:176, 2018
46. Ueno H, Kondo S, Yoshikawa S, Inoue K, Andre V, Tajimi M, Murakami H. A phase I and pharmacokinetic study of taladegib, a Smoothened inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs, 36:647-656, 2018
47. Seki Y, Fujiwara Y, Kohno T, Yoshida K, Goto Y, Horinouchi H, Kanda S, Nokihara H, Yamamoto N, Kuwano K, Ohe Y. Circulating cell-free plasma tumour DNA shows a higher incidence of EGFR mutations in patients with extrathoracic disease progression. ESMO Open, 3:e000292, 2018
48. Horinouchi H, Kubota K, Miyanaga A, Nakamichi S, Seike M, Gemma A, Yamane Y, Kurimoto F, Sakai H, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Tamura T, Ohe Y. Oral rehydration solution (OS-1) as a substitute of intravenous hydration after cisplatin administration in patients with lung cancer: a prospective multicenter trial. ESMO Open, 3:e000288, 2018
49. Tomuleasa C, Fuji S, Berce C, Onaciu A, Chira S, Petrushev B, Micu WT, Moisoiu V, Osan C, Constantinescu C, Pasca S, Jurj A, Pop L, Berindan-Neagoe I, Dima D, Kitano S. Chimeric Antigen Receptor T-Cells for the Treatment of B-Cell Acute Lymphoblastic Leukemia. Front Immunol, 9:239, 2018
50. Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, Yang JJ, Yamamoto N, Ahn MJ, Takahashi T, Yamanaka T, Kemner A, Roychowdhury D, Paolini J, Usari T, Wilner KD, Goto K. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol, 36:1405-1411, 2018
51. Ebata T, Shimoi T, Bun S, Miyake M, Yoshida A, Shimomura A, Noguchi E, Yonemori K, Shimizu C, Fujiwara Y, Narita Y, Tamura K. Efficacy and Safety of Pazopanib for Recurrent or Metastatic Solitary Fibrous Tumor. Oncology, 94:340-344, 2018
52. Ohmoto A, Morizane C, Kubo E, Takai E, Hosoi H, Sakamoto Y, Kondo S, Ueno H, Shimada K, Yachida S, Okusaka T. Germline variants in pancreatic cancer patients with a personal or family history of cancer fulfilling the revised Bethesda guidelines. J Gastroenterol, 2018
53. Kondo S, Sasaki M, Hosoi H, Sakamoto Y, Morizane C, Ueno H, Okusaka T. Incidence and risk factors for venous thromboembolism in patients with pretreated advanced pancreatic carcinoma. Oncotarget, 9:16883-16890, 2018
54. Sato J, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Ohe Y. Long-term survival without surgery in NSCLC patients with synchronous brain oligometastasis: systemic chemotherapy revisited. J Thorac Dis, 10:1696-1702, 2018
55. Kato M, Nakamura H, Nagai M, Kubo T, Elzawahry A, Totoki Y, Tanabe Y, Furukawa E, Miyamoto J, Sakamoto H, Matsumoto S, Sunami K, Arai Y, Suzuki Y, Yoshida T, Tsuchihara K, Tamura K, Yamamoto N, Ichikawa H, Kohno T, Shibata T. A computational tool to detect DNA alterations tailored to formalin-fixed paraffin-embedded samples in cancer clinical sequencing. Genome Med, 10:44, 2018
56. Shimoi T, Yoshida M, Kitamura Y, Yoshino T, Kawachi A, Shimomura A, Noguchi E, Yunokawa M, Yonemori K, Shimizu C, Kinoshita T, Ichimura K, Fukuda T, Fujiwara Y, Tamura K. TERT promoter hotspot mutations in breast cancer. Breast Cancer, 25:292-296, 2018
57. Bun S, Yonemori K, Akagi T, Noguchi E, Shimoi T, Shimomura A, Yunokawa M, Shimizu C, Fujiwara Y, Makino Y, Hayashi Y, Tamura K. Feasibility of olanzapine, multi acting receptor targeted antipsychotic agent, for the prevention of emesis caused by continuous cisplatin- or ifosfamide-based chemotherapy. Invest New Drugs, 36:151-155, 2018
58. Sasaki Y, Iwasa S, Okazaki S, Goto M, Kojima Y, Naganuma A, Nagashima K, Nagai Y, Hirano H, Honma Y, Takashima A, Kato K, Hamaguchi T. A phase II study of combination therapy with oral S-1 and cisplatin in elderly patients with advanced gastric cancer. Gastric Cancer, 21:439-445, 2018
59. Niikura N, Shimomura A, Fukatsu Y, Sawaki M, Ogiya R, Yasojima H, Fujisawa T, Yamamoto M, Tsuneizumi M, Kitani A, Watanabe J, Matsui A, Takahashi Y, Takashima S, Shien T, Tamura K, Saji S, Masuda N, Tokuda Y, Iwata H. Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis. Breast Cancer Res Treat, 167:81-87, 2018
60. Kato T, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, Tao L, Yu W, Khaznadar T, Tajima K, Shibata M, Seki A, Yamamoto N. Erlotinib Plus Bevacizumab Phase ll Study in Patients with Advanced Non-small-Cell Lung Cancer (JO25567): Updated Safety Results. Drug Saf, 41:229-237, 2018
61. Ohara S, Kanda S, Okuma H, Goto Y, Horinouchi H, Fujiwara Y, Nokihara H, Ito Y, Yamamoto N, Usui K, Homma S, Ohe Y. Effect of sequential chemoradiotherapy in patients with limited-disease small-cell lung cancer who were ineligible for concurrent therapy: a retrospective study at two institutions. Jpn J Clin Oncol, 48:82-88, 2018
62. Akiyoshi K, Hamaguchi T, Yoshimura K, Takahashi N, Honma Y, Iwasa S, Takashima A, Kato K, Yamada Y, Onodera H, Takeshita S, Yasui H, Sakai G, Akatsuka S, Ogawa K, Horita Y, Nagai Y, Shimada Y. A Prospective, Multicenter Phase II Study of the Efficacy and Feasibility of 15-minute Panitumumab Infusion Plus Irinotecan for Oxaliplatin- and Irinotecan-refractory, KRAS Wild-type Metastatic Colorectal Cancer (Short Infusion of Panitumumab Trial). Clin Colorectal Cancer, 17:e83-e89, 2018
63. Inagaki C, Shimoi T, Okuma H, Kitano A, Shimomura A, Noguchi E, Kodaira M, Yunokawa M, Yonemori K, Shimizu C, Yoshida A, Fujiwara Y, Tamura K. A case of heavily pretreated metastatic cardiac angiosarcoma treated successfully using eribulin. Anticancer Drugs, 29:97-101, 2018
64. Kato K, Ura T, Koizumi W, Iwasa S, Katada C, Azuma M, Ishikura S, Nakao Y, Onuma H, Muro K. Nimotuzumab combined with concurrent chemoradiotherapy in Japanese patients with esophageal cancer: A phase I study. Cancer Sci, 109:785-793, 2018
65. Ishiwata T, Iwasawa S, Ebata T, Fan M, Tada Y, Tatsumi K, Takiguchi Y. Inhibition of Gli leads to antitumor growth and enhancement of cisplatin-induced cytotoxicity in large cell neuroendocrine carcinoma of the lung. Oncol Rep, 39:1148-1154, 2018
